Abstract
This case report highlights the significance of a multidisciplinary approach in the management of patients with repaired membranous ventricular septal defect (VSD) who develop postoperative arrhythmias. We present the case of a young female who experienced symptomatic episodes of supraventricular tachycardia following VSD repair. Through electrophysiological study and radiofrequency ablation, multiple tachycardia substrates were identified and successfully ablated. This report underscores the importance of combining surgical repair, electrophysiological evaluation and intervention to achieve optimal outcomes in this specific patient population.
Keywords: Arrhythmia, adult congenital heart disease, electrophysiological study, radiofrequency ablation
Congenital heart disease (CHD) encompasses a wide spectrum of structural abnormalities affecting the cardiovascular system. Advances in medical and surgical interventions have significantly improved survival rates among individuals with CHD. However, the long-term consequences and associated complications of repaired CHD remain an ongoing challenge.
In this case report, we present a unique clinical scenario involving a patient with repaired ventricular septal defect (VSD) who presented with recurrent arrhythmias. The investigation of this case led to the identification of a multiple tachycardia substrate and coronary sinus diverticulum, a rare anatomical variant, as the underlying etiology for the arrhythmic manifestations. We describe the diagnostic and therapeutic interventions employed, which resulted in successful resolution of the patient’s symptoms.
CASE REPORT
A 17-year-old girl was referred to our arrhythmia center for electrophysiological study for recurrent paroxysmal episodes of regular palpitations. She had medical history of recently repaired VSD. Physical examination, transthoracic echocardiography (TTE) and biochemical tests were unremarkable. There was no family history of cardiac arrhythmia. Baseline electrocardiogram (ECG) showed sinus rhythm, pre-excited QRS with delta wave polarity suggestive of left posterior accessory pathway (Fig. 1).
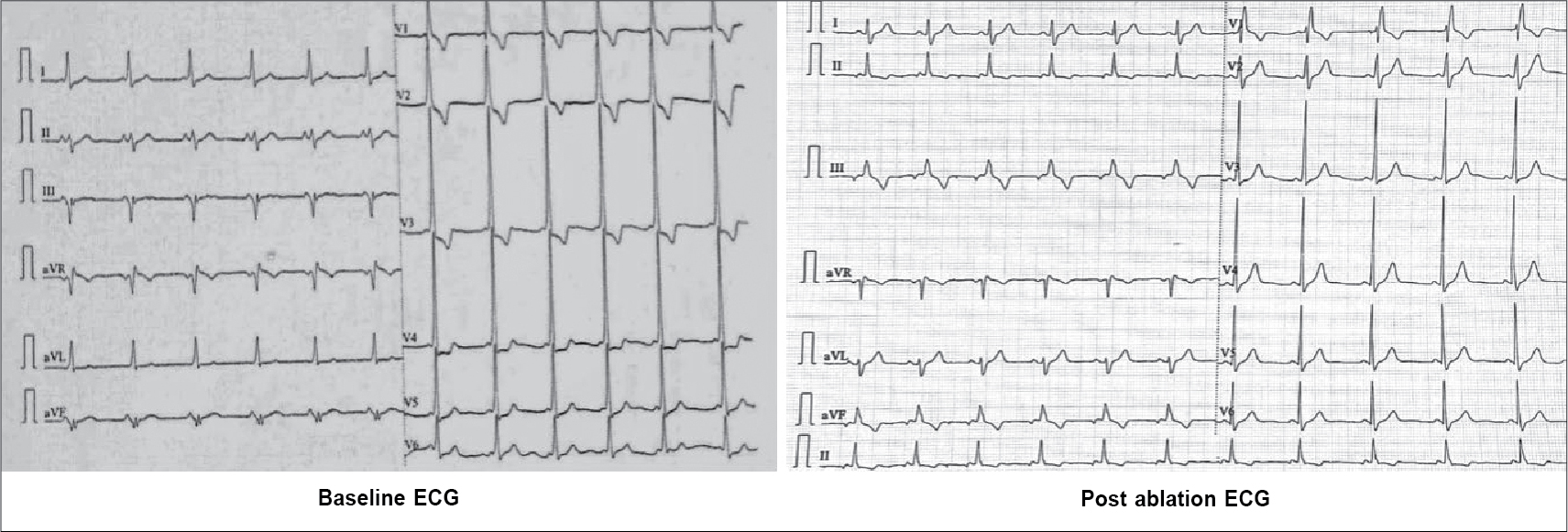
Figure 1. ECG (left) at baseline diagnostic of posteroseptal accessory pathway, ECG (right) after successful ablation of pathway with memory T waves in inferior leads.
After written informed consent, patient was taken for electrophysiological study. Two quadripolar diagnostic catheters were advanced under fluoroscopy to His bundle region and right ventricular apex. One decapolar catheter was advanced into the coronary sinus. During the electrophysiological study at baseline, atrial-His bundle (AH) and His-ventricular (HV) intervals were 85 ms and 3 ms in sinus rhythm, respectively. There were no atrioventricular (AV) conduction abnormalities. A narrow complex tachycardia (tachycardia cycle length [TCL] = 400 ms) was easily induced during catheter placement with following features: initiation of the tachycardia with a critical AH interval, fixed 1:1 ventriculoatrial (VA) conduction, concentric retrograde activation with VA interval of 37 ms, a post-pacing interval (PPI, 576 ms)–TCL (406 ms) >115 (170 ms) and ventricular overdrive pacing resulted in a VAHV response. His synchronized and early premature ventricular contraction (PVC) did not reset the tachycardia. His synchronized premature atrial contraction (PAC) also failed to reset the tachycardia. The tachycardia was reproducible and consistent (Fig. 2). After confirming the diagnosis of typical AV nodal re-entrant tachycardia (AVNRT) a decision was taken for slow pathway ablation. Using a steerable ablation catheter, with the help of intracardiac electrograms (EGMs) and using fluoroscopy, the region of the slow pathway was identified. Radiofrequency applications were made in the region of the slow pathway while constantly monitoring temperature, impedance, ECG and intracardiac EGMs, monitoring for fast junctional conduction and radiofrequency energy was halted if there was evidence of VA block. Radiofrequency ablation resulted in a junctional rhythm with intact AV conduction, which is a typical response. The AV nodal slow pathway was successfully modified (Fig. 3).
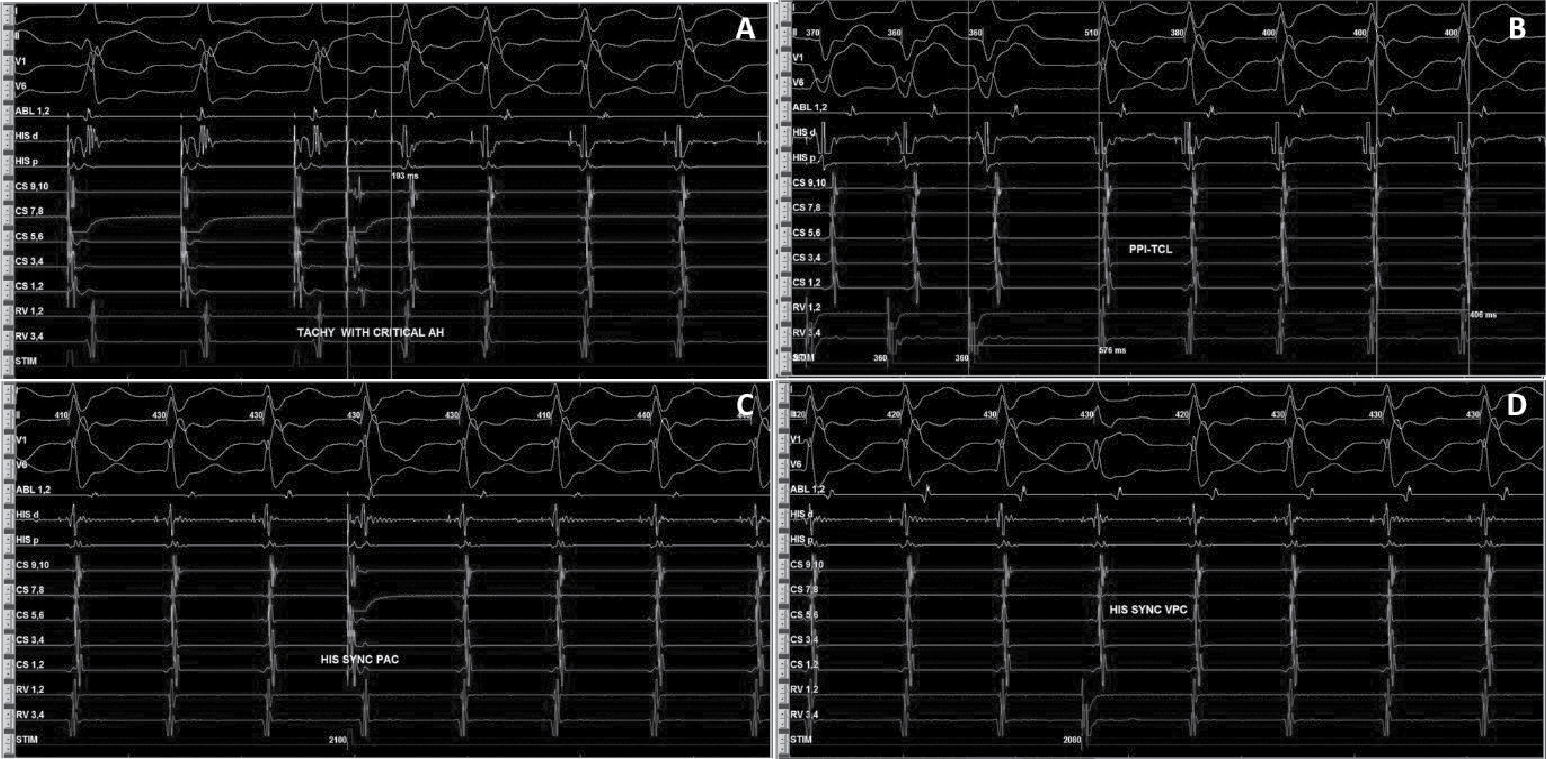
Figure 2. Intracardiac EGMs showing tachycardia induction with AH jump (A), RV overdrive pacing (B), and response of His synchronous PVCs (C) and PACs (D) diagnostic of typical slow-fast AVNRT.
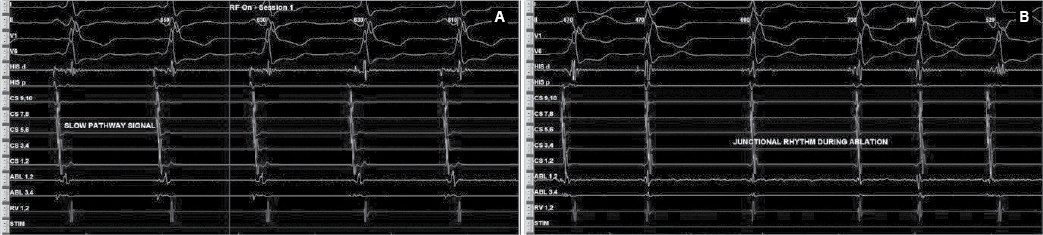
Figure 3. Slow pathway signals (A) at rightward inferior extension showed fused signals because of underlying pathway. Junctional rhythm (B) in response of radiofrequency energy.
Later on, during programmed atrial stimulation with burst pacing, patient developed another broad complex tachycardia (TCL = 220 ms) with 1:1 AV relationship. The tachycardia was hemodynamically unstable and led to ventricular fibrillation and required direct current cardioversion (DCCV) with 200J (Fig. 4). With the help of steerable ablation catheter, earliest atrial activation during ventricular pacing and earliest ventricular signal in sinus rhythm was mapped at the septal tricuspid annulus (RA mapping) and septal mitral annulus (LA mapping). Afterwards coronary sinus angiogram was taken, which showed a coronary sinus diverticulum near the decapolar coronary sinus 5-6 electrodes (Fig. 5). Radiofrequency applications were delivered at the neck of diverticulum that leads to disappearance of pre-excitation (Fig. 6). Afterwards aggressive programmed stimulation done on and off with isoproterenol that did not induce any atrial arrhythmias.
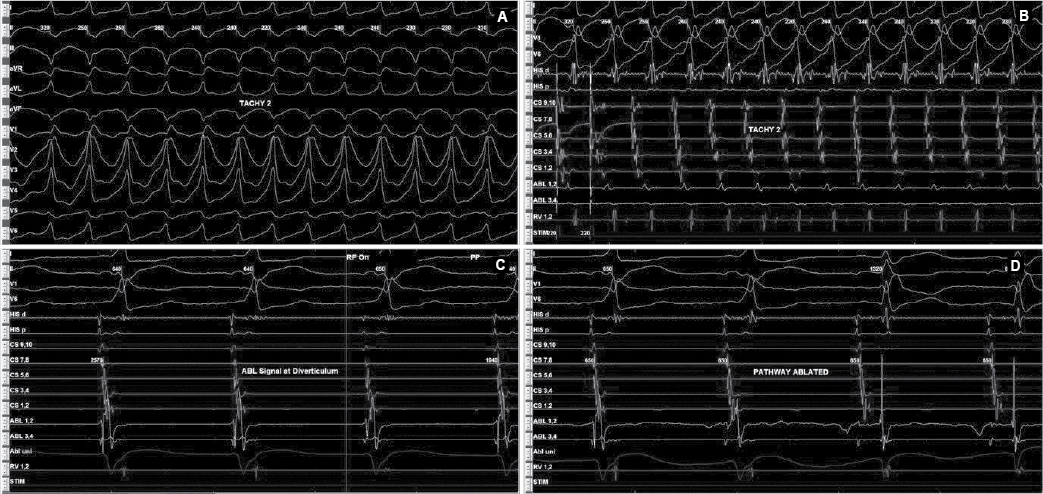
Figure 4. Antidromic tachycardia (A and B) involving posteroseptal pathway. EGMs recorded (C) inside diverticulum neck that was ablated successfully (D).
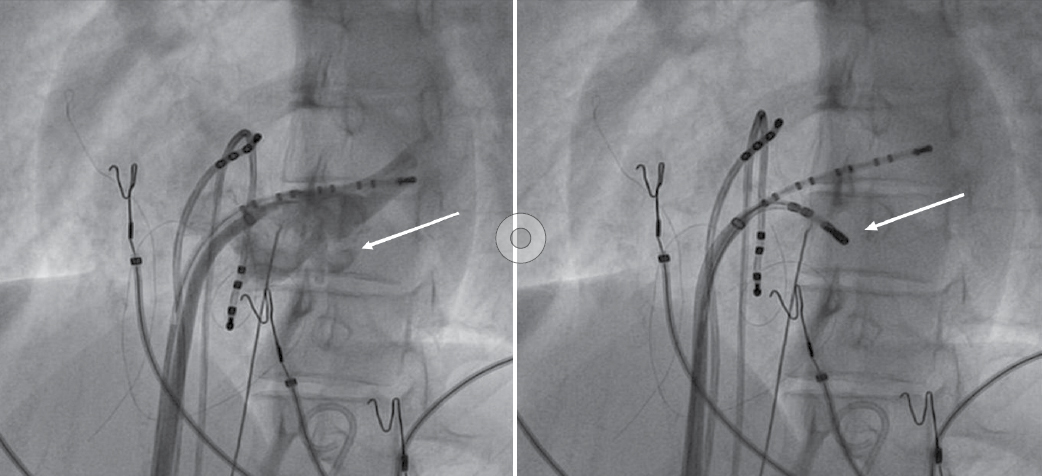
Figure 5. Coronary sinus angiogram (left) showing coronary sinus diverticulum and site of successful ablation (right).
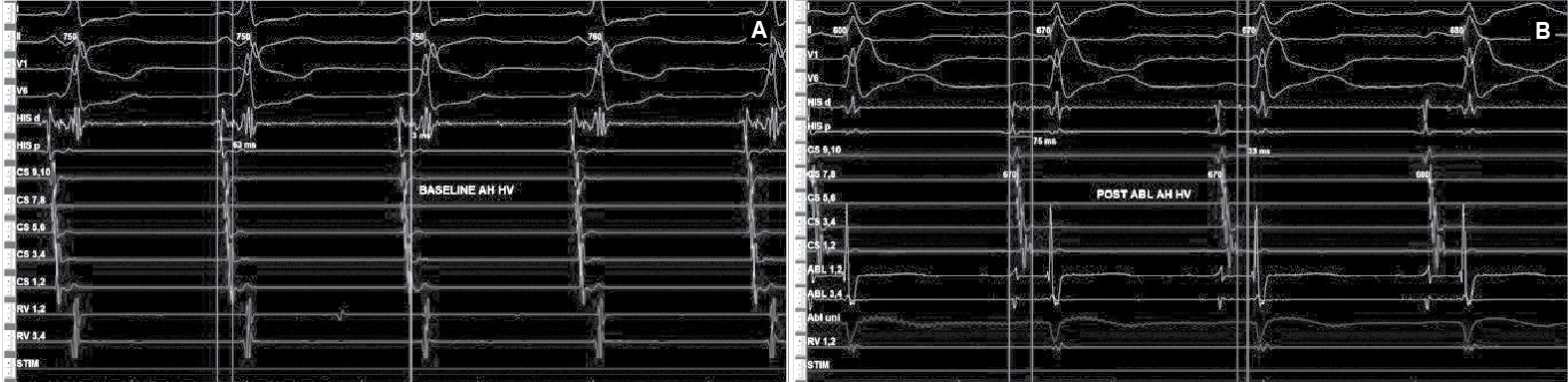
Figure 6. Intracardiac intervals baseline (A) showed HV interval of 3 ms that was normalized (B) after successful accessory pathway ablation.
During the follow-up, the patient remained free of symptoms and was not required to take antiarrhythmic agents. We did not find any conduction trouble on the ECG during the follow-up.
DISCUSSION
Coronary sinus diverticula are rare congenital anomalies. These are venous pouches at the pericardial aspect of posterior ventricular septum with neck draining into the proximal coronary sinus. They also contain accessory pathway at the neck of diverticula. Radiofrequency catheter ablation appears to be safe and curative in this setting. These diverticulae may be associated with other congenital cardiac abnormalities like membranous VSD and subaortic membranes.1-6
Surgical series suggest they may be present in 6% to 9% of patients with posteroseptal accessory pathways with symptoms severe enough to warrant surgery.7 In patients undergoing transesophageal echocardiography (TEE) following failed attempts at ablation of a posteroseptal accessory pathway, coronary sinus diverticulae were identified in 13% of cases. So, it is reasonable to do a coronary sinus venogram in case of posteroseptal or left posterior accessory pathway.
The posteroseptal accessory pathway is responsible for AV reciprocating tachycardia. Orthodromic re-entrant tachycardia mediated by posteroseptal pathway mimicking AVNRT, can be differentiated by different pacing maneuvers. However, the two may co-exist as seen in our case. Secondly, it is important to note that when accelerated junctional rhythm occurs during slow pathway ablation, the presence of retrograde atrial activation is reassuring, yet when a retrograde accessory pathway is present, the reassurance would be potentially false.
CONCLUSION
It is important to predict the accessory pathway location from the delta wave polarity. In patients with left posterior and posteroseptal accessory pathways coronary sinus venogram is recommended to identify coronary venous anomaly. Multiple arrhythmias can co-exist, so one needs to identify all the possible substrates.
Acknowledgment
We would like to acknowledge Mr Brijesh Yadav and Mr Mandeep Singh, Clinical Support, St. Jude Medical, Abbott India for their clinical assistance during the procedure.
This study has not received funding from any source.
Conflict of interest: None.
REFERENCES
- Saad EB, Marrouche NF, Cole CR, Natale A. Simultaneous epicardial and endocardial mapping of a left-sided posteroseptal accessory pathway associated with a large coronary sinus diverticulum: successful ablation by transection of the diverticulum’s neck. Pacing Clin Electrophysiol. 2002;25(10):1524-6.
- Sun Y, Arruda M, Otomo K, Beckman K, Nakagawa H, Calame J, et al. Coronary sinus-ventricular accessory connections producing posteroseptal and left posterior accessory pathways: incidence and electrophysiological identification. Circulation. 2002;106(11):1362-7.
- Al Fagih A, Al Zahrani G, Al Hebaishi Y, Dagriri K, Al Ghamdi SA. Coronary sinus diverticulum as a cause of resistant posteroseptal pathway ablation. J Saudi Heart Assoc. 2011;23(1):41-4.
- Morin DP, Parker H, Khatib S, Dinshaw H. Computed tomography of a coronary sinus diverticulum associated with Wolff-Parkinson-White syndrome. Heart Rhythm. 2012;9(8):1338-9.
- Raatikainen MJ, Pedersen AK. Catheter ablation of a difficult accessory pathway guided by coronary sinus venography and 3D electroanatomical mapping. 2010;12(8):1200-1.
- Veloor HP, Lokhandwala Y. A 2-year-old child with coronary sinus diverticulum and Wolff-Parkinson-White syndrome. Cardiol Young. 2013;23(2):274-6.
- Bennett DH, Hall MCS. Coronary sinus diverticulum containing posteroseptal accessory pathway. Heart. 2001;
86:539.