Published in IJCP
December 2023
Review Article
Role of Linagliptin in Type 2 Diabetes Mellitus Therapy: An Indian Perspective
December 11, 2023 | Rakesh Sahay, Ganapathi Bantwal, Shubhankar Chowdhary
Diabetes & Endocrinology Internal Medicine
Abstract
Given the rising prevalence and significant health and economic burdens associated with type 2 diabetes mellitus (T2DM) globally, especially in low- and middle-income countries, exploring effective treatment options is crucial. In this review, we have reviewed the role of linagliptin therapy in managing T2DM in the Indian context. The review specifically delves into the pharmacokinetic and pharmacodynamic properties of linagliptin, emphasizing its potential benefits in the context of the unique characteristics of Asian T2DM patients. The study provides valuable insights into the strategic use of linagliptin in the treatment landscape of T2DM, offering a comprehensive and all-encompassing approach to its clinical implications in various patient settings.
Keywords: Linagliptin, DPP-4 inhibitors, type 2 diabetes mellitus, gliptins
As per the International Diabetes Federation (IDF) Atlas 10th edition, 537 million adults (20-79 years) are living with diabetes. Over 3 in 4 adults with type 2 diabetes mellitus (T2DM) live in low- and middle-income countries. In South-East Asia, 1 in 11 adults are living with diabetes, which is expected to rise to 113 million by 2030 and 151 million by 2045. India accounts for 1 in 7 of all adults living with diabetes worldwide.1 As per the Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) study published in July 2023, the overall weighted prevalence of diabetes was 11.4%. Prediabetes 15.3% and generalized obesity 28.6%. All metabolic noncommunicable diseases (NCDs), including diabetes, were reported to be more frequent in urban than rural areas.2
T2DM is a chronic condition in which inadequate glycemic control is associated with several macro- and microvascular complications, including renal impairment, increased risk of coronary artery disease, stroke, high levels of low-density lipoprotein cholesterol and peripheral vascular disease.3 Microvascular complications of diabetes may also include end-stage renal disease (ESRD) and retinopathy. Considering the prevalence and severity of complications associated with inadequate glycemic control, attaining tight glycemic control is an important step in diabetes management.4 With India topping the list of individuals living with diabetes, health care costs associated with diabetes are high in terms of direct costs as well as loss of productivity related to chronic disability and premature mortality.4
Various pharmacological approaches are in use to improve glucose homeostasis through different modes of action, including sulfonylureas, biguanides, alpha-glucosidase inhibitors, thiazolidinediones, sodium-glucose co-transporter 2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors.5 DPP-4 inhibitors are a class of oral antidiabetic drugs (OADs) that increase the levels of glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP), which maintains glucose homeostasis by increasing insulin secretion in the pancreas, in turn reducing postprandial and fasting hyperglycemia.6 Currently, there are five DPP-4 inhibitors, including sitagliptin, saxagliptin, linagliptin, alogliptin, teneligliptin, evogliptin and vildagliptin. Despite the same mode of action and efficacy in terms of optimizing blood glucose levels, the various gliptins have differences in their pharmacodynamic and pharmacokinetic properties, which make them clinically relevant for different groups of patients.
Linagliptin is a DPP-4 inhibitor with several actions, including insulin secretion, reduced glucagon production, delayed gastric emptying, promotes satiety and decreased appetite.7 It is one of the most extensively studied OADs and has been extensively evaluated in a clinical trial program comprising more than 8,500 patients from different races and/or ethnic backgrounds.8
This article is an expert consensus on the place of linagliptin therapy in the T2DM treatment and management landscape in Indian experts.
GLYCEMIC EFFICACY OF LINAGLIPTIN
Asian T2DM patients are characterized by early age of diagnosis, long duration of disease, a high-risk for renal dysfunction and a beta-cell insufficiency. Hence, DPP-4 inhibitors are known to possess a therapeutic advantage over other drug classes in these patients, including those with low body mass index (BMI). Linagliptin enhances prandial insulin secretion and suppresses glucagon with a low risk of hypoglycemia.9
In a study by Kawamori et al (2012) assessing the efficacy of linagliptin versus placebo, a 1.2% change in glycated hemoglobin (HbA1c) was reported in the linagliptin group.10
In another study, linagliptin displayed superior glucose-lowering activity compared to placebo and alpha-glucosidase inhibitors such as voglibose.11 Figure 1 shows the comparative glucose-lowering efficacy of DPP-4 inhibitors.12
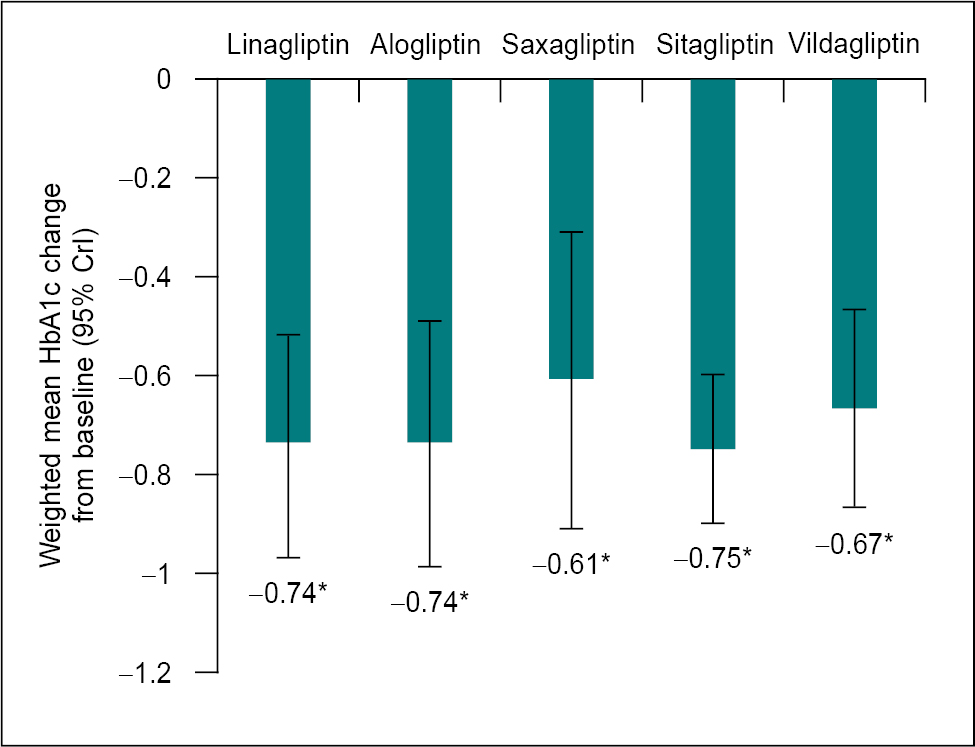
Figure 1. Glucose-lowering efficacy of DPP-4 inhibitors.12
*Statistically significant versus comparator: monotherapy versus placebo, DPP-4i plus metformin versus metformin, DPP-4i plus SU versus SU, DPP-4i plus metformin plus SU versus metformin plus SU, DPP-4i plus pioglitazone versus pioglitazone, DPP-4i plus insulin versus insulin, pioglitazone versus pioglitazone, DPP-4i plus insulin versus insulin.
CrI = Credible interval; DPP-4i = Dipeptidyl peptidase-4 inhibitor; SU = Sulfonylurea.
PLACE IN THERAPY: INITIATION
In a randomized controlled trial by Del Prato et al, linagliptin reduced HbA1c by 1.01% (p < 0.0001) in patients with baseline HbA1c ≥9.0%. In the same study, it was suggested that patients who underwent treatment for 24 weeks attained a reduction in HbA1c of ≥0.5%. These results prove that monotherapy with linagliptin has produced significant, clinically meaningful results in glycemic control and sustained effects accompanied by enhanced parameters of the beta-cell function.13
Studies on Asian subjects with T2DM and suboptimal glycemic control with metformin therapy showed that the mean change in HbA1c after 24 weeks of treatment with linagliptin was -0.79%, with similar adverse event profiles in the linagliptin and placebo group.14,15
PLACE IN THERAPY: INTERCHANGE
In patients who are unable to take metformin, linagliptin has proven to be a valuable alternative option. Linagliptin can improve glycemic control, especially in cases where metformin is inappropriate. It was seen in several randomized, controlled clinical trials in T2DM patients with inadequate glycemic control in whom metformin was inappropriate; monotherapy with linagliptin for 18 weeks led to a clinically meaningful lowering of HbA1c and fasting plasma glucose (FPG).16-18
PLACE IN THERAPY: INTENSIFICATION
Linagliptin as Second-line Therapy
As a second-line therapy, linagliptin leads to clinically relevant glycemic control when given to individuals with T2DM who had inadequate glycemic control (HbA1c ≥7.5% to ≤10%) with metformin alone. Forst et al, in a 2010 study, showed that linagliptin 5 mg led to a 0.73% (± 0.14) lowering in HbA1c.19
For patients inadequately controlled on metformin alone, linagliptin 5 mg once daily over 24 weeks brings a significant and clinically relevant improvement in glycemic control as seen through pre- and postprandial glucose levels and HbA1c levels. Linagliptin 5 mg leads to a 25% change in 2-hour postprandial glucose, a significant change as postprandial glucose state in patients with T2DM is related to endothelial dysfunction and hence the risk of cardiovascular complications.20
Inagaki et al (2013) evaluated once-daily linagliptin 5 mg as add-on therapy to an OAD (biguanide, glinide, glitazones, sulfonylurea or alpha-glucosidase inhibitors), the decline in HbA1c levels was seen throughout the study period for the background therapy groups receiving linagliptin. This suggests that linagliptin is a safe and tolerable alternative as second-line therapy over other OADs.21 Even though sulfonylureas have good glycemic-lowering ability, they are also associated with hypoglycemic events. In comparison, DPP-4 inhibitors like linagliptin with good glucose-lowering efficacy without hypoglycemic side effects, making it a clinically stable choice for second-line therapy.22
|
In T2DM management, acute hypoglycemic events and the OAD’s effect on body weight are two important things to consider. Linagliptin has a low propensity for acute hypoglycemic events and a neutral effect on body weight. Linagliptin is an effective, well-tolerated and logical choice for patients with T2DM who are inadequately treated with metformin alone.
|
Linagliptin Compared to Combination Therapy
Linagliptin has been shown to be an important treatment option for individuals with inadequate glycemic control despite ongoing combination therapy. Owens et al (2011) showed in a randomized study that when linagliptin was administered to T2DM patients on therapy with metformin and sulfonylurea, it led to a reduction in HbA1c (29.2% vs. 8.1% placebo, p < 0.0001), FPG and improvements in homeostasis model assessment of beta-cell function.23
Results from Phase III studies on Asian patients, including Japanese, have exhibited clinically relevant improvements in glycemic control, improved beta-cell function,19,24 and a good safety profile with linagliptin 5 mg as monotherapy or in combination with other OADs.15,20,23,25 In the double-blind active comparator-controlled study by Kawamori et al (2012), linagliptin demonstrated superior glucose-lowering efficacy and comparable safety and tolerability to both placebo and voglibose.10
Linagliptin is also beneficial as triple combination therapy with empagliflozin and metformin in patients with type 2 diabetes. Studies have shown that 24 weeks of treatment with the triple combination reduced the HbA1c significantly.26,27
Linagliptin Combination with Other Oral Antidiabetics
In view of various challenges posed by the complex pathophysiology of T2DM, the involvement of multiple organs, including the heart, blood vessels, nerves, eyes and kidneys, and the deterioration of beta-cell function, the T2DM management is shifting towards a more proactive and aggressive treatment approach, which involves the initiation of combination pharmacotherapy immediately following diagnosis. It has been shown that initial combination therapy with linagliptin and metformin was superior to metformin monotherapy in improving glycemic control, with a similar safety and tolerability profile, no weight gain and a low risk of hypoglycemia. It was also seen that initial combination treatment with linagliptin and metformin combination produced a more rapid improvement in glycemic control compared to the stepwise approach from metformin monotherapy to linagliptin and metformin combination therapy.18
An early, aggressive reduction of hypoglycemia in newly diagnosed patients can elicit sustained disease remission. In the 10-year follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) by von Eynatten et al, early, intensive pharmacotherapy led to long-term benefits in reducing the incidence of microvascular complications and cardiovascular events.28 In a study by Mu et al, linagliptin and metformin combination significantly improved glycemic control in treatment-naïve Asian patients with T2DM and inadequate glycemic control.29
Linagliptin can be safely given as an add-on treatment to glimepiride to improve glycemic variability and control without enhancing the risk of hypoglycemia in patients with hepatocyte nuclear factor 1α (HNF-1α) or maturity-onset diabetes of the young type 3.30
Treatment with linagliptin led to noninferior glycemic control and considerably reduced risk of hypoglycemia compared to insulin glargine in long-term care and individuals with long-term diabetes.31Additionally, linagliptin can be safely added to basal insulin therapy to lead to considerable improvement in glycemic control compared to placebo without increasing hypoglycemia or body weight.32
The addition of linagliptin to insulin early in the therapy led to a reduction in the daily insulin dose along with improved glycemic control and sustained effect over the long duration of therapy.33
Linagliptin vs. Other DPP-4 Inhibitors
A direct comparison revealed that all DPP-4 inhibitors were significantly more effective compared to placebo in attaining a greater mean reduction from baseline in HbA1c, and a higher proportion of individuals met the target of HbA1c <7%.12 Table 1 depicts the difference in HbA1c, hypoglycemic events and weight gain among different DPP-4 inhibitors.34
|
Table 1. Difference in Diabetes Efficacy and Safety Parameters among DPP-4 Inhibitors
|
| |
Sitagliptin
|
Vildagliptin
|
Saxagliptin
|
Linagliptin
|
|
HbA1c lowering
|
WMD –0.74%
|
WMD –0.60%
|
WMD –0.57%
|
WMD –0.68%
|
|
Weight gain
|
WMD 0.22 kg
|
WMD 0.26 kg
|
WMD 0.14 kg
|
WMD 0.15 kg
|
|
Hypoglycemia
|
OR 2.94
|
OR 0.85
|
OR 1.19
|
OR 0.93
|
|
The mean reduction in HbA1c in patients with severe renal insufficiency35
|
-
|
0.5% at week 12 and 0.7% at week 52
|
0.45% and 0.32% at weeks 12 and 52, respectively
|
–0.60% at week 12 and –0.72% at week 52
|
WMD = Weighted mean difference; OR = Odds ratio.
The results of a Bayesian network meta-analysis of 58 randomized controlled trials showed that amongst all the doses, linagliptin 5 mg reduced the levels of 5 mg most, compared to linagliptin 10 mg and 0.5 mg. It has been proven that 5 mg/day of linagliptin for 12 to 24 weeks significantly reduced FPG.36 Furthermore, in a network meta-analysis of randomized clinical trials comparing sitagliptin with linagliptin, the proportion of patients achieving HbA1c <7% was higher in the linagliptin group.37
Patients treated with either sitagliptin or vildagliptin resulted in a clinically meaningful increase in mean body weight compared to placebo of 0.70 kg and 0.83 kg, respectively. However, there was no significant difference in mean change in body weight for alogliptin or linagliptin versus placebo (Fig. 2). Linagliptin is the only DPP-4 inhibitor with a statistically significant lesser chance of patients having a hypoglycemic event compared with placebo.12
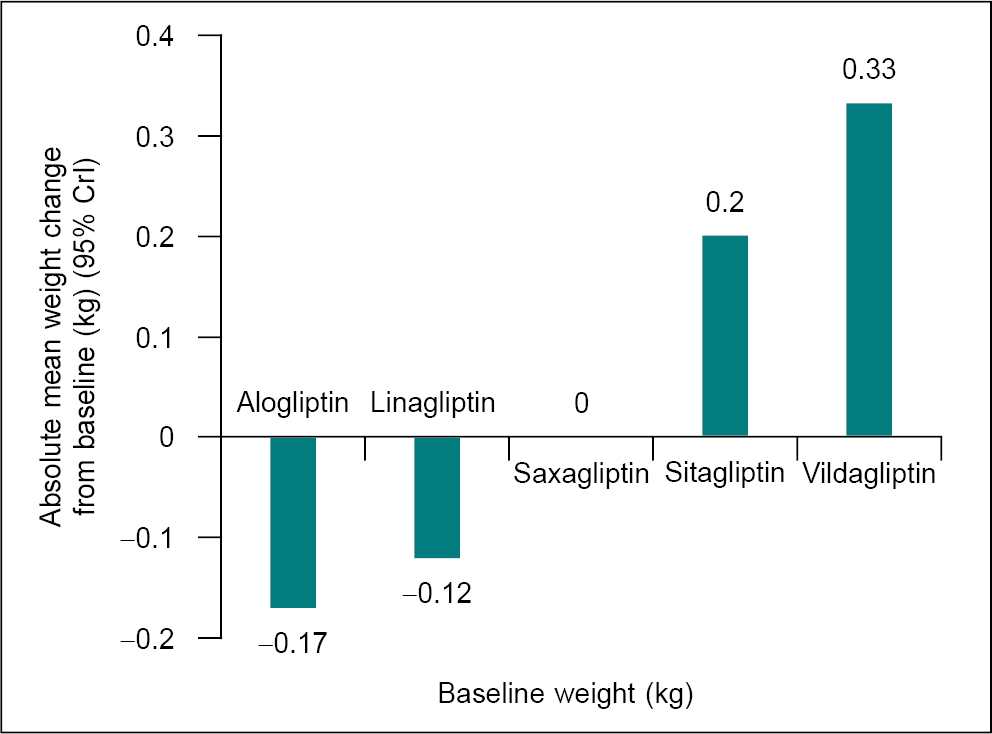
Figure 2. Absolute treatment effect mixed treatment comparisons-Absolute mean weight change from baseline (following 54 weeks of treatment), kg (95% credible interval CrI).
(Data sourced from Craddy P, et al. Diabetes Ther. 2014;5(1):1-41.12)
The results of the study by DeFronzo et al showed that after a 24-week treatment with the combination, the reduction with SGLT2 inhibitor/DPP-4 inhibitor was superior to those with SGLT2 inhibitor or DPP-4 inhibitor individually as add-on to metformin.38
DPP-4 inhibitor and SGLT2 inhibitor combination are safe, effective, tolerable and rationale cost-effective in patients with uncontrolled blood glucose on therapy with metformin alone, intolerance to metformin, increased baseline HbA1c, overweight or obesity and diabetic hypertensive, congestive heart failure, atherosclerotic cardiovascular disease (CVD), and renal dysfunction patients.39
LINAGLIPTIN IN PATIENTS WITH VASCULAR COMPLICATIONS
The presence of clinical features such as microalbuminuria and hypertension is the key to assessing the risk of cardiovascular and renal outcomes. Vascular complications are a primary challenge in the management of T2DM. It was seen in the UKPDS trial that one-fourth of the study population developed microalbuminuria within 10 years of being diagnosed with T2DM. The American Diabetes Association (ADA) guidelines and the Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines40,41 for diabetes and chronic kidney disease (CKD) on T2DM management recommend reducing the risk or delaying the progression of kidney diseases through strict normalization of glycemic control. Renal impairment acts as a challenge in the selection of appropriate OADs in managing T2DM.28
According to a study, linagliptin can improve endothelial and neurovascular microvascular function and is related to reduced markers of inflammation in patients with type 2 diabetes.42
Managing Patients with Renal Impairment
A characteristic feature of linagliptin is that the primary route of elimination is via the nonrenal route, with 5% of the dose being excreted via the kidneys (Fig. 3). Further, linagliptin requires no dose adjustment when given to patients with renal issues. In comparison, all other DPP-4 inhibitors are predominantly cleared by renal excretion as well as dose adjustment is required, especially in the case of creatinine clearance <50 mL/min and those with ESRD requiring dialysis. A study by McGill et al (2013) showed that in patients with type 2 diabetes and severe renal impairment, after 1-year treatment period, only a few patients experienced severe hypoglycemia in linagliptin and placebo groups. The linagliptin group showed superior mean HbA1c and FPG at week 12, and patients undergoing changes in 1 or more daily glucose-lowering background therapy was similar between linagliptin and placebo. No significant change was seen in the rate of adverse events, and no change was seen in the average estimated glomerular filtration rate (eGFR) values by clinically meaningful relevance.43
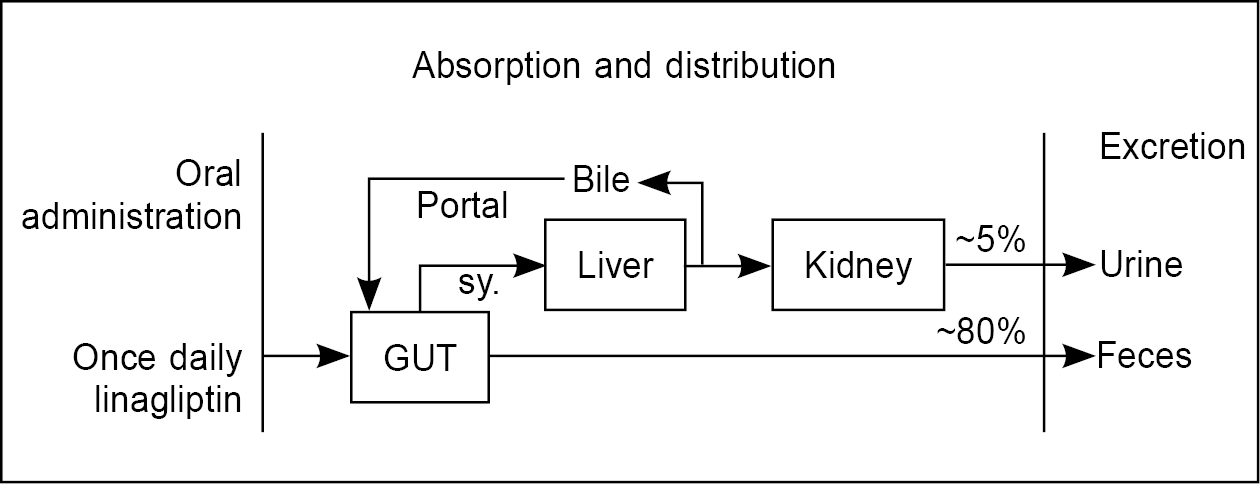
Figure 3. Elimination of linagliptin.
Von Eynatten et al, in a study on 512 patients with T2DM at high renal and vascular risk, linagliptin demonstrated a significant and clinically meaningful reduction in HbA1c and FPG compared to placebo. Linagliptin treatment also leads to minor alterations in urine albumin-creatinine ratio.28 Linagliptin, when given across three renal function categories, achieved a consistent reduction in HbA1c levels; normal (-0.63%; p < 0.0001), mild renal impairment (-0.67%; p < 0.0001) and moderate renal impairment (-0.53%; p < 0.01). The efficacy of linagliptin remained stable across all groups, and it proved to be an effective, well-tolerated, and convenient treatment in individuals with T2DM and mild to moderate renal impairment.44
The CARMELINA cohort comprised patients with T2DM and CVD, and/or CKD, contradictory to the restricted renal risk population of other cardiovascular outcomes (CVOTs). It is the first DPP-4 inhibitor CVOT in a substantial proportion of patients with renal dysfunction and/or characterized microalbuminuria at baseline. Table 2 depicts the renal safety of DPP-4 inhibitors as shown across different CVOTs. CARMELINA CVOT identified and utilized the opportunity to include a significant number of patients with CKD along with patients with CVD, as these patients will remain apt for linagliptin at a single dose, irrespective of the decline in renal function (Table 2).45
|
Table 2. Renal Safety of DPP-4 Inhibitors
|
| |
Sitagliptin
|
Saxagliptin
|
Alogliptin
|
Linagliptin
|
|
Renal safety composite
|
Studies not reported
|
Neutral
|
|
Renal function
|
Studies show moderately increased risk
|
|
Albuminuria
|
Studies show modest benefit
|
Studies not reported
|
Studies show modest benefit
|
Managing Patients with Cardiovascular Disorders
A comparison with other DPP-4 inhibitors has shown that linagliptin has a modest effect on blood pressure while others, such as saxagliptin and sitagliptin, have small to neutral effects on blood pressure.28 Further, linagliptin was well tolerated and effective when added to the treatment regimen of patients with T2DM and coronary artery disease. It was not related to an increased incidence of cardiac adverse events. The CARMELINA trial was a cardiovascular event-driven, placebo-controlled, double-blind, randomized clinical trial conducted between August 2013 and January 2018 in 27 countries across Asia, Europe, Latin America and North America. In the CARMELINA trial, it was seen that linagliptin did not raise the risk of cardiovascular events or hypoglycemia in older patients with T2DM and established CVD. Linagliptin did not cause the risk of hospitalization for heart failure across all age groups.46
Compared to CARMELINA, TECOS was a global clinical trial performed in a usual care setting among patients with type 2 diabetes and established CVD; the DPP-4 inhibitor did not affect rates of major atherosclerotic cardiovascular events. However, the study enrolled patients with moderate hyperglycemia and excluded those with renal insufficiency.47
Linagliptin in Patients with Liver Disease
Liver disease has a high prevalence in people with T2DM. Linagliptin has been noted to be effective and well-tolerated in people with T2DM and liver disease. Linagliptin brought about a change in HbA1c from baseline in individuals with hepatic disorders, -0.75% compared to -0.20% with placebo.21 Dose adjustment with linagliptin is not needed in patients with hepatic impairment.48
SPECIAL SITUATIONS
Linagliptin effectively lowered hyperglycemia in Asian patients with uncontrolled T2DM, irrespective of age, BMI, renal function or ethnic subgroups and was well-tolerated9 (Table 316,49-55).
|
Table 3. Linagliptin Use Across Different Patient Profiles with T2DM
|
|
Patient category
|
Glucose-lowering efficacy
|
Safety
|
Benefits
|
|
Elderly patients (≥65 years)
|
The mean change in HbA1c was -0.64% (241 patients)16
|
Well-tolerated
No risk of hypoglycemia or other drug-related side effects.49,50
|
Achieve individualized glycemic targets with the least chance of risk.
Improves glucose control in patients on stable insulin therapy.51
|
|
Treatment-naïve patients
|
Significant and clinically meaningful improvement of glycemic control.52
|
Excellent safety profile52
|
Improves parameters of β-cell function.52
|
|
Pediatric patients
|
Dose-dependent reduction in mean HbA1c of 0.48% and 0.63% with linagliptin 1 and 5 mg, respectively; linked to a corresponding lowering in mean FPG of 5.6 and 34.2 mg/dL.53
|
Well-tolerated53
|
Dose-dependent DPP-4 inhibition accompanied by a corresponding lowering of HbA1c and FPG levels.53
|
|
Hospitalized surgical patients
|
Effective in patients undergoing noncardiac surgery with mild to moderate hyperglycemia (blood glucose <11.1 mmol/L).54
|
Safe55
Fewer hypoglycemic events compared to basal-bolus insulin.54
|
Safe and effective choice as an alternative to multi-dose insulin therapy.54
|
CLINICAL SAFETY OF LINAGLIPTIN
The clinical safety of linagliptin has been evaluated in more than 4,000 patients with type 2 diabetes. In a placebo-controlled clinical study, nasopharyngitis occurred in about 5% of the linagliptin group of patients; and more often in the linagliptin group compared to placebo. Other adverse events associated with linagliptin were hypersensitivity and myalgia.55
Hypoglycemic events are rare during treatment with linagliptin. In fact, in a study of linagliptin versus placebo, monotherapy led to hypoglycemic events occurring in 0.6% of the linagliptin group and 0.3% of the placebo group.13 In another placebo-controlled study of linagliptin monotherapy, no episodes of hypoglycemia were reported in patients with type 2 diabetes.56 DPP-4 inhibitors, in general, have a neutral effect on weight. It was reported in a study that weight loss of 0.15, 0.57 and 12.7 kg was seen with linagliptin 1 mg, 5 mg and 10 mg, respectively.19 However, combination therapy with metformin led to a decrease in body weight in a 24-week study.57 Linagliptin did not cause any significant changes in electrocardiographic parameters. A randomized, placebo-controlled, double-blind, four-period crossover study showed that linagliptin had the ability to prolong the QT interval at therapeutic and subtherapeutic doses.58 Linagliptin was well-tolerated, and no clinically meaningful electrocardiographic changes or relevant changes in other safety parameters were observed.
Figure 4 shows the treatment algorithm for managing type 2 diabetes patients with combination therapies.
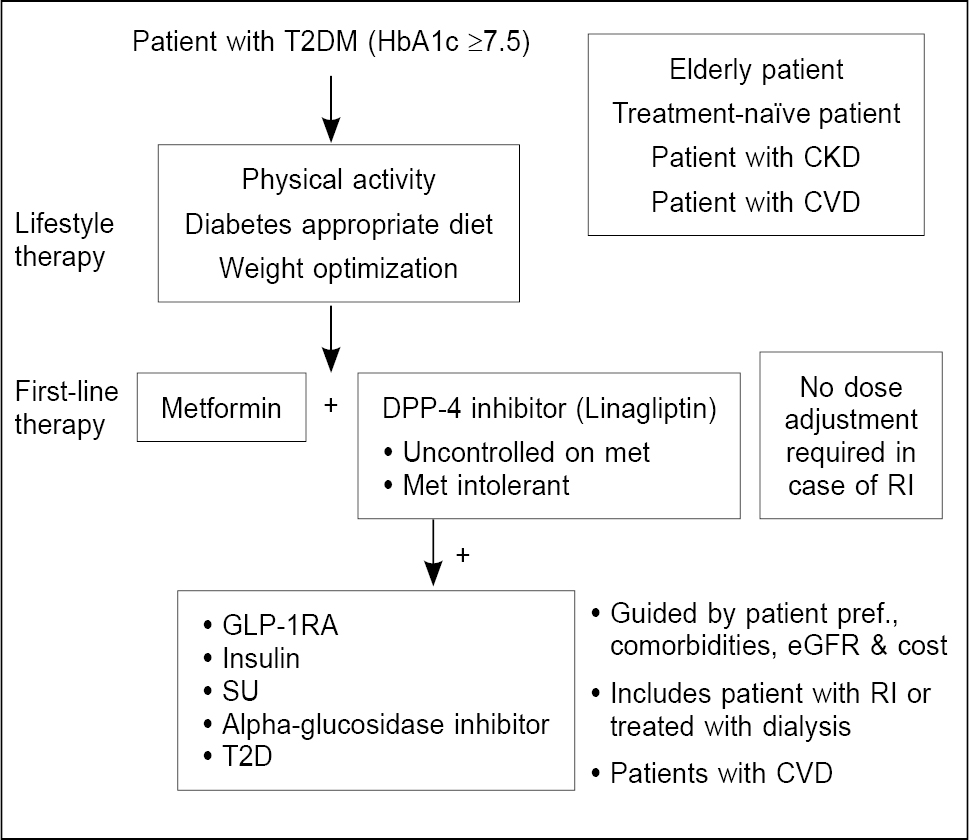
Figure 4. Algorithm for the place of linagliptin in T2DM management.
T2DM = Type 2 diabetes mellitus; HbA1c = Glycated hemoglobin; CKD = Chronic kidney disease; CVD = Cardiovascular disease; DPP-4 = Dipeptidyl peptidase-4; RI = Renal impairment; GLP-1RA = Glucagon-like peptide-1 receptor agonists; SU = Sulfonylurea; eGFR = Estimated glomerular filtration rate.
CONCLUSION
In recent years, DPP-4 inhibitors have emerged as a promising option for the management of T2DM. Among them, linagliptin has a unique pharmacokinetic and pharmacodynamic profile featured by target-mediated nonlinear pharmacokinetics and a large safety window, more than 100 times the recommended daily dose. Linagliptin has shown clinically meaningful glucose-lowering efficacy and good tolerability as monotherapy or second-/third-line therapy across several race groups.
Numerous clinical trials have demonstrated the efficacy of linagliptin in reducing HbA1c levels and improving glycemic control, both as monotherapy and in combination with other oral antidiabetic agents. It has also shown a favorable effect on beta-cell function, making it particularly beneficial for Asian populations with beta-cell insufficiency. Another crucial advantage of linagliptin is its cardiovascular safety profile, as seen in the CARMELINA trial, setting it apart from other DPP-4 inhibitors, making it a favorable choice for patients with CVDs and/or kidney impairment.
Evidence suggests that the use of linagliptin in combination with other OADs, such as metformin, would prove to be a well-tolerated and effective treatment option for T2DM patients. Overall, it would provide a comprehensive approach to managing T2DM by addressing both CVD risk factors as well as hyperglycemia, ultimately improving patient outcomes and quality of life. The combination of DPP-4 inhibitors and SGLT2 inhibitors is also beneficial as second-line therapy in T2DM patients uncontrolled on metformin therapy.
|
Expert Recommendations
Place in Therapy: Initiation
· Linagliptin may be used as first-line therapy in persons in whom metformin is contraindicated.
· Linagliptin may be used as part of initial dual oral therapy in persons with HbA1c >7.5%.
· Linagliptin may be used as part of initial triple oral therapy if persons with HbA1c >8.5% at presentation.
Place in Therapy: Interchange
· Linagliptin may be used as monotherapy in persons in whom metformin is not tolerated.
· Linagliptin may be used to replace any other oral antidiabetic agents/injectable GLP-1RA if the drug is deemed unsafe or is not tolerated.
Place in Therapy: Intensification
· Linagliptin may be used as add-on therapy if pre-existing metformin monotherapy is inadequate in achieving desired HbA1c targets.
· Linagliptin may be used as add-on therapy if pre-existing dual therapy is inadequate in achieving desired HbA1c targets.
· Linagliptin may be co-prescribed with insulin to reduce insulin requirements to minimize glycemic variability and provide renoprotective benefits.
Special Situations
· Linagliptin is the preferred drug of choice in persons with renal impairment, including those on dialysis, irrespective of eGFR status.
· Linagliptin may be used in persons with moderate hepatic impairment.
· Linagliptin has been shown to have beneficial effects in persons with foot infections.
· Linagliptin may be used in persons with chronic heart disease, irrespective of ejection fraction.
· Linagliptin is the preferred drug of choice in persons at high risk of hypoglycemia.
Caveats and Considerations
· Linagliptin does not need any dose titration based on renal or hepatic status.
· Linagliptin can be safely prescribed to persons who are unable to undergo frequent investigations or follow-up regularly.
· Linagliptin can be co-prescribed with every glucose-lowering drug except other gliptins and GLP-1RA.
|
REFERENCES
- International Diabetes Federation. IDF Atlas. 10th Edition, 2022.
- Anjana RM, Unnikrishnan R, Deepa M, Pradeepa M, Tandon N, Das AK, et al; ICMR-INDIAB Collaborative Study Group. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023;11(7):474-89.
- Khurana L, Durand EM, Gary ST, Otero AV, Dumais KM, Beck J, et al. Mechanisms for improving diabetes patient-provider communication through optimal use of e-clinical technologies. Patient Prefer Adherence. 2019;13:981-92.
- Challenges in diabetes management: Glycemic control, medication adherence, and healthcare costs. AJMC. Aug 21, 2017. Available from: https://www.ajmc.com/view/challenges-in-diabetes-management-article. Accessed July 18, 2023.
- Makrilakis K. The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: when to select, what to expect. Int J Environ Res Public Health. 2019;16(15):2720.
- Kasina SVSK, Baradhi KM. Dipeptidyl peptidase IV (DPP IV) inhibitors. [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542331/
- Tella SH, Akturk HK, Rendell M. Linagliptin for the treatment of type 2 diabetes. Diabetes Manag. 2014;4(1):85-101.
- Sarashina A, Friedrich C, Crowe S, Patel S, Graefe-Mody U, Hayashi N, et al. Comparable pharmacodynamics, efficacy, and safety of linagliptin 5 mg among Japanese, Asian and white patients with type 2 diabetes. J Diabetes Investig. 2016;7(5):744-50.
- Ning G, Bandgar T, Hehnke U, Lee J, Chan JCN. Efficacy and safety of linagliptin in 2681 Asian patients stratified by age, obesity, and renal function: a pooled analysis of randomized clinical trials. Adv Ther. 2017;34(9):2150-62.
- Kawamori R, Inagaki N, Araki E, Watada H, Hayashi N, Horie Y, et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab. 2012;14(4):348-57.
- Del Prato S, Taskinen MR, Owens DR, von Eynatten M, Emser A, Gong Y, et al. Efficacy and safety of linagliptin in subjects with type 2 diabetes mellitus and poor glycemic control: pooled analysis of data from three placebo-controlled phase III trials. J Diabetes Complications. 2013;27(3):274-9.
- Craddy P, Palin HJ, Johnson KI. Comparative effectiveness of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a systematic review and mixed treatment comparison. Diabetes Ther. 2014;5(1):1-41.
- Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13(3):258-67.
- Zeng Z, Choi DS, Mohan V, Emser A, Siddiqui K, Gong Y, et al. Efficacy and safety of linagliptin as monotherapy or add-on treatment in Asian patients with suboptimal glycemic control: a pooled analysis. Curr Med Res Opin. 2015;31(1):99-106.
- Wang W, Yang J, Yang G, Gong Y, Patel S, Zhang C, et al. Efficacy and safety of linagliptin in Asian patients with type 2 diabetes mellitus inadequately controlled by metformin: a multinational 24-week, randomized clinical trial. J Diabetes. 2016;8(2):229-37.
- Barnett AH, Patel S, Harper R, Toorawa R, Thiemann S, von Eynatten M, et al. Linagliptin monotherapy in type 2 diabetes patients for whom metformin is inappropriate: an 18-week randomized, double-blind, placebo-controlled phase III trial with a 34-week active-controlled extension. Diabetes Obes Metab. 2012;14(12):1145-54.
- Wu D, Li L, Liu C. Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Obes Metab. 2014;16(1):30-7.
- Haak T, Meinicke T, Jones R, Weber S, von Eynatten M, Woerle HJ. Initial combination of linagliptin and metformin in patients with type 2 diabetes: efficacy and safety in a randomised, double-blind 1-year extension study. Int J Clin Pract. 2013;67(12):1283-93.
- Forst T, Uhlig-Laske B, Ring A, Graefe-Mody U, Friedrich C, Herbach K, et al. Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled type 2 diabetes. Diabet Med. 2010;27(12):1409-19.
- Taskinen MR, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi KA, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13(1):65-74.
- Inagaki N, Watada H, Murai M, Kagimura T, Gong Y, Patel S, et al. Linagliptin provides effective, well-tolerated add-on therapy to pre-existing oral antidiabetic therapy over 1 year in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):833-43.
- Gillani SW, Moosvi AF. Clinical review: Safety and efficacy comparison between sulfonylureas and dipeptidyl peptidase-4 inhibitors as second-line therapies in type 2 diabetes mellitus. Curr Pharm Des. 2020;26(34):4315-22.
- Owens DR, Swallow R, Dugi KA, Woerle HJ. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011;28(11):1352-61.
- Forst T, Uhlig-Laske B, Ring A, Ritzhaupt A, Graefe-Mody U, Dugi KA. The oral DPP-4 inhibitor linagliptin significantly lowers HbA1c after 4 weeks of treatment in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2011;13(6):542-50.
- Gomis R, Espadero RM, Jones R, Woerle HJ, Dugi KA. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13(7):653-61.
- Søfteland E, Meier JJ, Vangen B, Toorawa R, Maldonado-Lutomirsky M, Broedl UC. Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24-week randomized, double-blind, parallel-group trial. Diabetes Care. 2017;40(2):201-9.
- Tinahones FJ, Gallwitz B, Nordaby M, Götz S, Maldonado-Lutomirsky M, Woerle HJ, et al. Linagliptin as add-on to empagliflozin and metformin in patients with type 2 diabetes: two 24-week randomized, double-blind, double-dummy, parallel-group trials. Diabetes Obes Metab. 2017;19(2):266-74.
- von Eynatten M, Gong Y, Emser A, Woerle HJ. Efficacy and safety of linagliptin in type 2 diabetes subjects at high risk for renal and cardiovascular disease: a pooled analysis of six phase III clinical trials. Cardiovasc Diabetol. 2013;12:60.
- Mu Y, Pan C, Fan B, Hehnke U, Zhang X, Zhang X, et al. Efficacy and safety of linagliptin/metformin single-pill combination as initial therapy in drug-naïve Asian patients with type 2 diabetes. Diabetes Res Clin Pract. 2017;124:48-56.
- Christensen AS, Hædersdal S, Støy J, Storgaard H, Kampmann U, Forman JL, et al. Efficacy and safety of glimepiride with or without linagliptin treatment in patients with HNF1A diabetes (maturity-onset diabetes of the young type 3): a randomized, double-blinded, placebo-controlled, crossover trial (GLIMLINA). Diabetes Care. 2020;43(9):2025-33.
- Umpierrez GE, Cardona S, Chachkhiani D, Fayfman M, Saiyed S, Wang H, et al. A randomized controlled study comparing a DPP4 inhibitor (linagliptin) and basal insulin (glargine) in patients with type 2 diabetes in long-term care and skilled nursing facilities: Linagliptin-LTC trial. J Am Med Dir Assoc. 2018;19(5):399-404.e3.
- Yki-Järvinen H, Rosenstock J, Durán-Garcia S, Pinnetti S, Bhattacharya S, Thiemann S, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥52-week randomized, double-blind study. Diabetes Care. 2013;36(12):3875-81.
- Katsuno T, Shiraiwa T, Iwasaki S, Park H, Watanabe N, Kaneko S, et al; TRUST2 study group. Benefit of early add-on of linagliptin to insulin in Japanese patients with type 2 diabetes mellitus: randomized-controlled open-label trial (TRUST2). Adv Ther. 2021;38(3):1514-35.
- Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother. 2012;46(11):1453-69.
- Thomas MC, Paldánius PM, Ayyagari R, Ong SH, Groop PH. Systematic literature review of DPP-4 inhibitors in patients with type 2 diabetes mellitus and renal impairment. Diabetes Ther. 2016;7(3):439-54.
- Ling J, Cheng P, Ge L, Zhang DH, Shi AC, Tian JH, et al. The efficacy and safety of dipeptidyl peptidase-4 inhibitors for type 2 diabetes: a Bayesian network meta-analysis of 58 randomized controlled trials. Acta Diabetol. 2019;56(3):249-72.
- Keshavarz K, Lotfi F, Sanati E, Salesi M, Hashemi-Meshkini A, Jafari M, et al. Linagliptin versus sitagliptin in patients with type 2 diabetes mellitus: a network meta-analysis of randomized clinical trials. Daru. 2017;25(1):23.
- DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38(3):384-93.
- Chenchula S, Varthya SB, Padmavathi R. Rationality, efficacy, tolerability of empagliflozin plus linagliptin combination for the management of type 2 diabetes mellitus: a systematic review of randomized controlled trials and observational studies. Curr Diabetes Rev. 2022;18(4):e100921196392.
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(Suppl 1):S11-63.
- National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-86.
- Baltzis D, Dushay JR, Loader J, Wu J, Greenman RL, Roustit M, et al. Effect of linagliptin on vascular function: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2016;101(11):4205-13.
- McGill JB, Sloan L, Newman J, Patel S, Sauce C, von Eynatten M, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013;36(2):237-44.
- Groop PH, Del Prato S, Taskinen MR, Owens DR, Gong Y, Crowe S, et al. Linagliptin treatment in subjects with type 2 diabetes with and without mild-to-moderate renal impairment. Diabetes Obes Metab. 2014;16(6):560-8.
- Schernthaner G, Wanner C, Jurišic-Eržen D, Guja C, Gumprecht J, Jarek-Martynowa IR, et al. CARMELINA: An important piece of the DPP-4 inhibitor CVOT puzzle. Diabetes Res Clin Pract. 2019;153:30-40.
- Cooper ME, Rosenstock J, Kadowaki T, Seino Y, Wanner C, Schnaidt S, et al; CARMELINA Investigators. Cardiovascular and kidney outcomes of linagliptin treatment in older people with type 2 diabetes and established cardiovascular disease and/or kidney disease: a prespecified subgroup analysis of the randomized, placebo-controlled CARMELINA trial. Diabetes Obes Metab. 2020;22(7):1062-73.
- Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232-42.
- Graefe-Mody U, Rose P, Retlich S, Ring A, Waldhauser L, Cinca R, et al. Pharmacokinetics of linagliptin in subjects with hepatic impairment. Br J Clin Pharmacol. 2012;74(1):75-85.
- Schernthaner G, Barnett AH, Patel S, Hehnke U, von Eynatten M, Woerle HJ. Safety and efficacy of the dipeptidyl peptidase-4 inhibitor linagliptin in elderly patients with type 2 diabetes: a comprehensive analysis of data from 1331 individuals aged ≥ 65 years. Diabetes Obes Metab. 2014;16(11):1078-86.
- Guo XH, Feng ZK, Xu LH. The efficacy and safety of linagliptin in elderly patients with type 2 diabetes: a pooled analysis of eight placebo-controlled clinical trials. Zhonghua Nei Ke Za Zhi. 2017;56(8):588-94.
- Ledesma G, Umpierrez GE, Morley JE, Lewis-D’ Agostino D, Keller A, Meinicke T, et al. Efficacy and safety of linagliptin to improve glucose control in older people with type 2 diabetes on stable insulin therapy: a randomized trial. Diabetes Obes Metab. 2019;21(11):2465-73.
- Wu W, Li Y, Chen X, Lin D, Xiang S, Shen F, et al. Effect of linagliptin on glycemic control in Chinese patients with newly-diagnosed, drug-naïve type 2 diabetes mellitus: a randomized controlled trial. Med Sci Monit. 2015;21:2678-84.
- Tamborlane WV, Laffel LM, Weill J, Gordat M, Neubacher D, Retlich S, et al. Randomized, double-blind, placebo-controlled dose-finding study of the dipeptidyl peptidase-4 inhibitor linagliptin in pediatric patients with type 2 diabetes. Pediatr Diabetes. 2018;19(4):640-8.
- Vellanki P, Rasouli N, Baldwin D, Alexanian S, Anzola I, Urrutia M, et al; Linagliptin Inpatient Research Group. Glycaemic efficacy and safety of linagliptin compared to a basal-bolus insulin regimen in patients with type 2 diabetes undergoing non-cardiac surgery: a multicentre randomized clinical trial. Diabetes Obes Metab. 2019;21(4):837-43.
- Freeman MK. Efficacy and safety of linagliptin (tradjenta) in adults with type 2 diabetes mellitus. P T. 2011;36(12):807-42.
- Heise T, Graefe-Mody EU, Hüttner S, Ring A, Trommeshauser D, Dugi KA. Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes Metab. 2009;11(8):786-94.
- Tradjenta (linagliptin), package insert. Ridgefield Conn.: Boehringer Ingelheim; July 2011. Available at: http://bidocs.boehringer-ingelheim.com. Accessed July 18, 2023.
- Ring A, Port A, Graefe-Mody EU, Revollo I, Iovino M, Dugi KA. The DPP-4 inhibitor linagliptin does not prolong the QT interval at therapeutic and supratherapeutic doses. Br J Clin Pharmacol. 2011;72(1):39-50.
|