Abstract
Sturge-Weber syndrome (SWS) is a phakomatosis involving the eyelids, conjunctiva, choroid and retina. The mechanism of glaucoma in SWS involves developmental anomaly of the anterior chamber angle and elevated episcleral venous pressure. Here, we present a case of Roach’s type II SWS with bilateral angle-closure glaucoma (ACG) in a young adult, which to the best of our knowledge, is the first-of-its-kind report in the literature. Though unusual, ACG may occur in young adults too. However, the underlying causes that make them develop the ACG are poorly understood. We also highlight the importance of comprehensive clinical examination and appropriate investigations for early detection and proper treatment of such young patients with ACG.
Keywords: Sturge-Weber syndrome, Roach’s type II variant, glaucoma, angle-closure glaucoma, young adult, laser peripheral iridotomy
Sturge-Weber syndrome (SWS) is a neurocutaneous disorder characterized by unilateral facial cutaneous venous dilation (port-wine stain), leptomeningeal capillary-venous malformation and vascular abnormalities of the eye. Vascular abnormalities can affect any portion of the eye, including the eyelid, orbit, conjunctiva, episclera, ciliary body, retina and choroid. Glaucoma occurs in 30% to 70% of individuals of SWS, is usually unilateral and often diagnosed in infancy, though it can also develop later.1 Theories regarding the mechanism of glaucoma in eyes with SWS are developmental anomalies of the anterior chamber angle and elevated episcleral venous pressure, each leading to aqueous outflow obstruction.2 The Roach scale is often used for the classification of SWS.3
Angle-closure glaucoma (ACG) is rare in young adults. The current knowledge of ACG in young patients needs to be improved. Young patients presenting with ACG are likely to have different etiologies, which are not very well understood.4
Here we report an unusual case of bilateral ACG in a 24-year-old man with unilateral Roach’s type II SWS.
Case Presentation
A 24-year-old man presented to us with headache, and decreased vision in his left eye (LE) for 6 months. He was on four antiglaucoma medications (bimatoprost 0.03%, timolol maleate 0.5%, brimonidine tartrate 0.2% and brinzolamide 1%) in his right eye (RE) for 6 months. He had undergone a diode laser cyclophotocoagulation in his LE 5 years ago elsewhere. He had no family history of glaucoma. He had a capillary hemangioma on the left half of his face involving the fifth nerve dermatome, which had been present since birth (Fig. 1). The patient had no history of seizures or paretic episodes and was not on systemic medications. He also provided reports of a magnetic resonance imaging (MRI) of the brain, which did not reveal any leptomeningeal involvement.
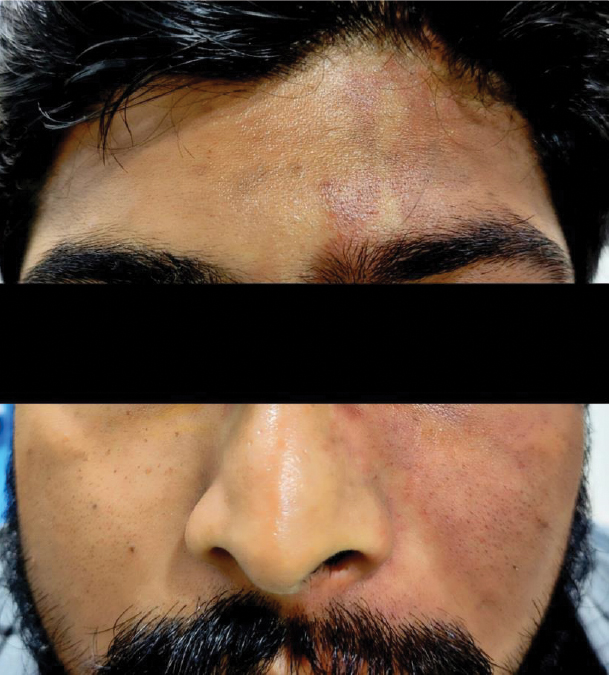
Figure 1. Port-wine stain on the left half of the face.
On examination, the corrected distance visual acuity was 20/20 in RE with no perception of light in LE. The intraocular pressure (IOP) was 23 mmHg and 19 mmHg on the Goldmann applanation tonometer in RE and LE, respectively. Anterior segment examination of RE revealed a clear cornea, shallow anterior chamber (Van Herick Grade 1), round reacting pupil and a clear crystalline lens (Fig. 2a). LE showed dilated tortuous episcleral vessels, clear cornea, shallow anterior chamber, relative afferent pupillary defect and a clear crystalline lens (Fig. 2b). Gonioscopy revealed Shaffer grade with 180 degrees synechial angle closure in RE and total synechial angle closure in LE. Undilated fundus examination showed a cup-disk ratio of 0.7 with no localized areas of neuroretinal rim loss and normal macula in RE (Fig. 2c) and glaucomatous optic atrophy in LE. The central corneal thickness was 481 µm and 478 µm in RE and LE, respectively. B-scan ultrasonography of both eyes was within normal limits (Fig. 2d). The axial length was 21.80 mm and 21.92 mm in RE and LE, respectively. Ultrasound biomicroscopy of BE showed no signs of a forward shift of the lens-iris diaphragm, anterior rotation of the ciliary process, choroidal effusion or ciliary body swelling.
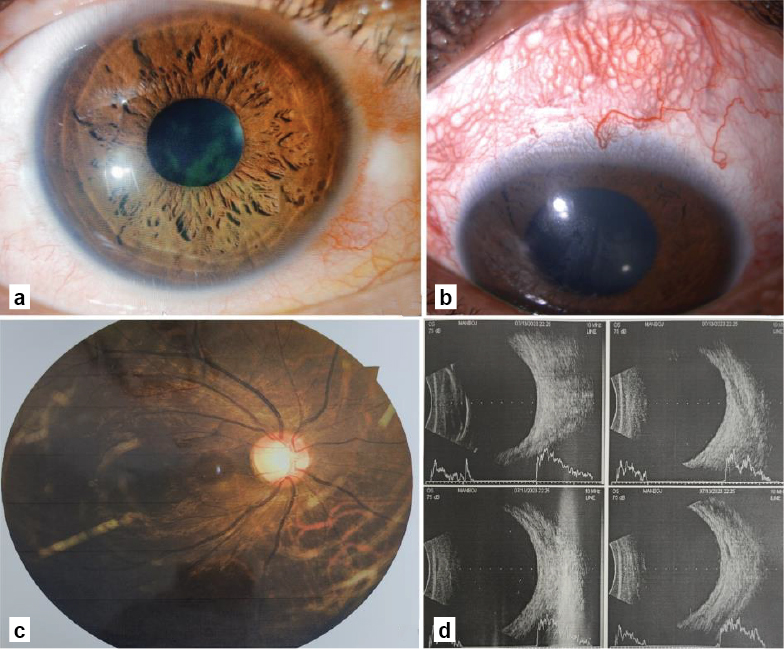
Figure 2. (a) Slit-lamp photograph of right eye; (b) Slit-lamp photograph of left eye showing dilated tortuous episcleral vessels; (c) Fundus photograph of right eye showing a cup-disk ratio of 0.7 with no localized areas of neuroretinal rim loss and normal macula and (d) B-scan ultrasonography within normal limits.
A provisional diagnosis of Roach’s type II SWS with bilateral ACG was made based on clinical findings. A neodymium-doped yttrium aluminum garnet (Nd:YAG) laser peripheral iridotomy (LPI) was performed in RE to relieve the pupillary block. One hour later, the IOP came down to 18 mmHg in RE. At 1 week follow-up, the IOP was 17 mmHg in RE, and the LPI was patent.
The RE visual field test was performed using Humphrey Field Analyzer (HFA), Swedish Interactive Threshold Algorithm (SITA) 24-2C program, which revealed a scotoma in the paracentral area (Fig. 3a). A dilated fundus examination revealed no additional findings. Optical coherence tomography (OCT) of RE showed a retinal nerve fiber layer thickness of 95 µm (average), 103 µm (superior quadrant) and 87 µm (inferior quadrant) (Fig. 3b). The ganglion cell complex also showed thinning inferiorly (Fig. 3c). The investigations established the diagnosis of unilateral SWS (Roach’s type II variant) with ACG, and the patient was continued on two antiglaucoma medications.
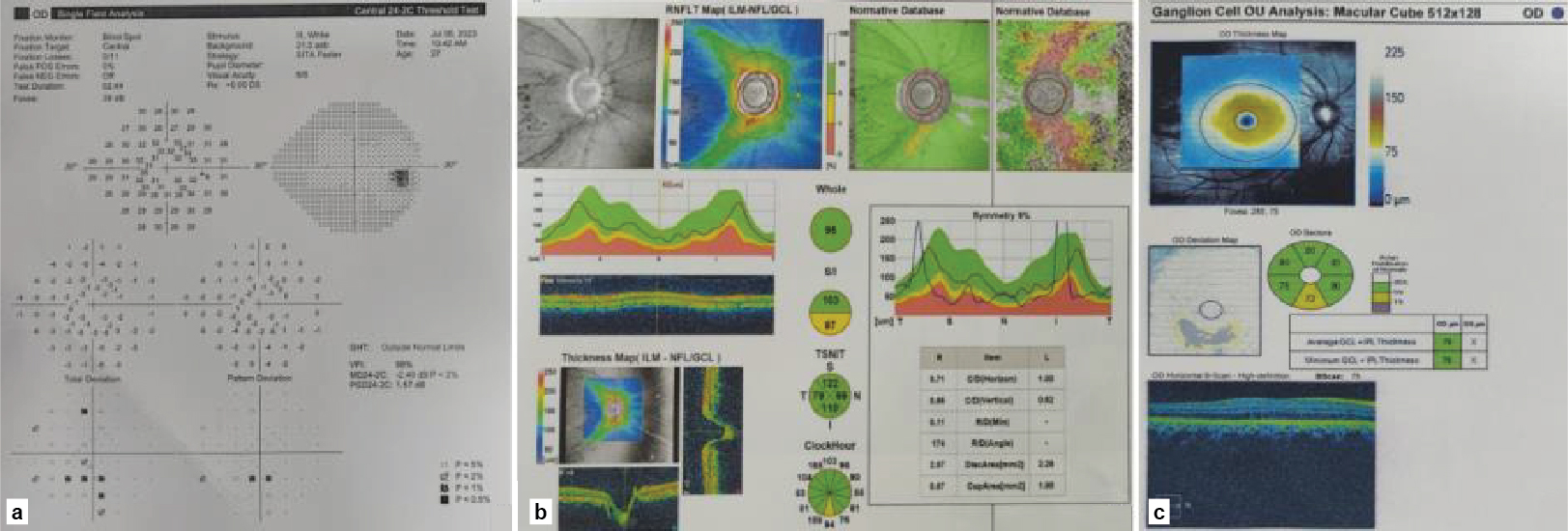
Figure 3. Right eye (a) Swedish Interactive Threshold Algorithm 24-2C program revealing a scotoma in the paracentral area; (b) OCT showing retinal nerve fiber layer thickness in different quadrants and (c) Ganglion cell complex thinning in the inferior quadrant.
DISCUSSION
Glaucoma in SWS is typically congenital, with buphthalmos related to chamber angle malformation, as in other types of congenital glaucoma. In juvenile and adult SWS, the alteration of trabecular meshwork Schlemm canal complex and persistence of Streeter primordial vascular plexus, leading to episcleral venous pressure increase, has been described as the cause of glaucoma development.2
ACG is a rare presentation in SWS, which has been reported as a consequence of ectopia lentis, posterior scleritis, chronic uveitis and alteration of the ciliary body configuration secondary to diffuse choroidal hemangioma.5-9 However, unlike our case, all these case reports had a unilateral secondary angle closure mechanism associated with SWS. In the present case, the patient had ACG in the contralateral eye, too and all possibilities of a secondary angle closure mechanism were ruled out with the help of investigations. The undiagnosed angle closure component superimposed with elevated episcleral venous pressure might in fact have been responsible for development of glaucomatous optic atrophy in the LE.
Another unique feature of this case report is the presentation of ACG in a young patient. There are limited studies about the etiologies of angle closure in young patients. Most of them were scattered cases except the ones by Gao et al and Ritch et al.4,10 Primary ACG, uveitis and anterior segment dysgenesis were the common three etiologies of ACG in the study by Gao et al, which accounted for 32.6%, 20.3% and 15.1% of the total patients (463 eyes), respectively. Other known etiologies included iridocorneal endothelial syndrome, neovascular glaucoma, nanophthalmos, retinitis pigmentosa, spherophakia, bestrophinopathy, persistent fetal vasculature, iridociliary cysts, congenital retinoschisis, Marfan’s syndrome, retinopathy of prematurity, familial exudative vitreoretinopathy, congenital retinal folds, Coat’s disease and neurofibromatosis.4 In our case, we carried out a comprehensive clinical examination and investigated systematically to rule out the known secondary etiologies of ACG.
CONCLUSION
To the best of our knowledge, this is the first report of a case of SWS associated with bilateral ACG in a young patient. A comprehensive clinical examination and appropriate investigations can identify most of the etiologies of ACG in young patients, which helps in early detection and timely treatment.
Acknowledgment
The manuscript has been read and approved by all the authors, the requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Conflict of Interest: No conflicts of interest.
Financial Disclosure:
No financial support and sponsorship.
The authors have no financial or proprietary interest in any material or method mentioned.
REFERENCES
- Sullivan TJ, Clarke MP, Morin JD. The ocular manifestations of the Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus. 1992;29(6):349-56.
- Celebi S, Alagöz G, Aykan U. Ocular findings in Sturge-Weber syndrome. Eur J Ophthalmol. 2000;10(3):239-43.
- Roach ES. Neurocutaneous syndromes. Pediatr Clin North Am. 1992;39(4):591-620.
- Gao F, Wang J, Chen J, Wang X, Chen Y, Sun X. Etiologies and clinical characteristics of young patients with angle-closure glaucoma: a 15-year single-center retrospective study. Graefes Arch Clin Exp Ophthalmol. 2021;259(8):2379-87.
- Moore DB, Reck SD, Chen PP. Angle closure glaucoma associated with ectopia lentis in a patient with Sturge-Weber syndrome. Eye (Lond). 2011;25(9):1235-6.
- Lee DH, Shin J, Seo JH, Byon IS, Jung JH, Lee JE. Mobile lens-induced angle closure glaucoma and rubeosis iridis in Sturge Weber syndrome. Int J Ophthalmol. 2015;8(5):1080-2.
- Maruyama I, Ohguro H, Nakazawa M. A case of acute angle-closure glaucoma secondary to posterior scleritis in patient with Sturge-Weber syndrome. Jpn J Ophthalmol. 2002;46(1):74-7.
- Lambiase A, Mantelli F, Mannino G, Recupero SM. An unusual case of acute glaucoma in Sturge-Weber syndrome. Eur J Ophthalmol. 2015;25(6):e103-5.
- Su WW. Acute primary angle-closure in Sturge-Weber syndrome. Am J Ophthalmol Case Rep. 2018;10:101-4.
- Ritch R, Chang BM, Liebmann JM. Angle closure in younger patients. Ophthalmology. 2003;110(10):1880-9.