Abstract
Autoimmune hemolytic anemia (AIHA) is a medical disorder where the immune system erroneously targets and destroys red blood cells (RBCs), causing a reduction in their longevity. This results in an inadequate supply of RBCs to carry oxygen, leading to symptoms like fatigue, weakness and jaundice. AIHA is categorized into warm and cold types based on the optimal temperature for antibody activity. A comprehensive grasp of the immunological mechanisms involved is essential for accurate diagnosis and effective management of this autoimmune condition. AIHA is a frequently missed diagnosis as patient are not fully investigated, and treated with vitamin B12, folic acid and iron in outpatient department.
Keywords: Autoimmune hemolytic anemia, reticulocyte count, lactate dehydrogenase, peripheral smear
Idiopathic autoimmune hemolytic anemia (IAIHA) refers to a subtype of autoimmune hemolytic anemia (AIHA) where the underlying cause of the immune system’s attack on red blood cells (RBCs) cannot be identified. This form of AIHA presents a diagnostic challenge, as it lacks a clear association with known triggers or underlying conditions.1
Patients with IAIHA experience the hallmark symptoms of AIHA, including fatigue, weakness and jaundice, stemming from the destruction of their own RBCs by autoantibodies.
CASE REPORT
A 39-year-old female presented to hospital on 20th October 2023 with complaint of abdominal pain since 6 to 7 days, predominant in epigastric and left upper quadrant, associated with generalized weakness, easy fatigability since last 15 days.
All the vitals were within normal limits. On general examination, there was pallor and icterus. On abdominal examination, the spleen was palpable 2 fingers below the costal margins.
There was no history of any drug intake or recent infection/fever.
Patient was treated empirically with parenteral vitamin B12 1,000 µg IV × 5 doses, oral folic acid 5 mg once daily and blood transfusion 1 unit packed cell volume. The patient was discharged on oral iron, B12 and folic acid.
Patient again presented after 2 months with similar complaints, with pallor and icterus and falling hemoglobin.
Detailed examination and investigation turned out to be a hemolytic anemia, with high indirect bilirubin, raised lactate dehydrogenase (LDH), high reticulocyte count, positive direct Coombs test and hemolysis on peripheral smear (Fig. 1). After thorough investigation on second admission, a diagnosis of idiopathic warm AIHA was made.
|
Investigations
|
| |
20-Oct-23
|
22-Oct-23
|
14-Dec-2023
|
6-Jan-23
|
18-Jan-23
|
|
Hb (g/dL) (12-15g/dL)
|
7.6
|
8.9
|
7.2
|
9.8
|
10.2
|
|
WBC(cells/cu mm)
(4,000-11,000 cells/cu mm)
|
6,800
|
7,200
|
9,400
|
7,400
|
8,200
|
|
Platelet (cells/cu mm)
(1,50,000-4,00,000 cells/cu mm)
|
1,97,000
|
2,12,000
|
2,02,000
|
2,30,000
|
2,32,300
|
|
Creatinine (mg/dL) (<1.2 mg/dL)
|
1.0
|
0.9
|
0.8
|
0.9
|
0.9
|
|
Upper GI endoscopy
|
Normal
|
|
|
|
|
|
Serum SGPT (U/L) (10-56 U/L)
|
78
|
74
|
|
43
|
|
|
Bilirubin (total/indirect)
|
2.3/1.8
|
|
2.8/2.2
|
1.1/0.9
|
1.0/0.8
|
|
LDH (U/L) (140-280 U/L)
|
623
|
|
1,142
|
212
|
198
|
|
dsDNA (0-29.9 IU/mL)
|
|
|
Negative
|
|
|
|
ANA - Immunofluorescence
(Normal <1:160 titer)
|
|
|
Negative (1:40 titer)
|
|
|
|
ESR (mm/1st hr) (0-20 mm/1st hr)
|
|
45
|
|
|
|
|
PSCM, reticulocyte count (RC)
|
|
|
Moderate anisocytosis, spherocytosis & nucleated RBC, RC-4.2%
|
|
|
|
HIV, HBV, HCV, EBV IgM
|
|
|
Negative
|
|
|
|
C-reactive protein
|
|
Negative
|
Negative
|
|
|
|
Direct Coombs test (DCT)
|
|
DCT+2
|
DCT+4
|
|
|
|
Ultrasonography abdomen
|
|
|
Splenomegaly, 16 cm length
|
|
|
Hb = Hemoglobin; WBC = White blood cell; GI = Gastrointestinal; SGPT = Serum glutamic-pyruvic aminotransferase; LDH = Lactate dehydrogenase; dsDNA = Double-stranded deoxyribonucleic acid; ANA = Antinuclear antibody; ESR = Erythrocyte sedimentation rate; PSCM = Peripheral smear cell morphology; RBC = Red blood cell; HIV = Human immunodeficiency virus; HBV = Hepatitis B virus; HCV = Hepatitis C virus; EBV = Epstein-Barr virus; IgM = Immunoglobulin M.
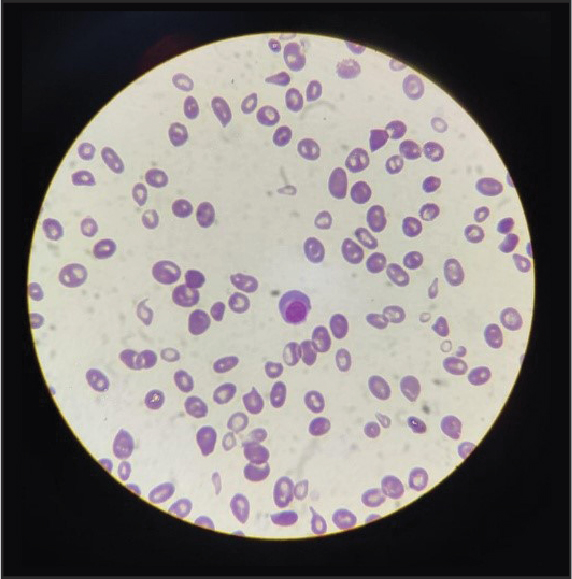
Figure 1. Peripheral smear.
Investigation
Diagnosis involves a comprehensive assessment, including blood tests such as the direct and indirect Coombs tests to detect the presence of antibodies on RBCs. Distinguishing IAIHA from secondary forms requires ruling out underlying causes such as infections, medications or other autoimmune disorders.2
Treatment
Treatment strategies for IAIHA often parallel those for other forms of AIHA. Corticosteroids remain a primary therapeutic choice, aiming to suppress the immune response and alleviate hemolysis. In more resistant cases, immunosuppressive agents or other advanced therapies may be considered to manage the condition.3
From 15th December 2023, patient was started on tablet prednisolone 20 mg once daily, for 2 weeks, followed by tapering dose 5 mg/2 week for next 4 weeks. By 15th January 2024, after 1 month of steroid, patient is currently on 10 mg prednisolone, having clinical improvement with resolution of easy fatigability, shortness of breath on exertion and recovery in laboratory signs of hemolysis. No side effects of steroids were noted during the course of treatment.
Prednisolone is planned to be slowly tapered over the next 1 month and to be stopped after a total of 8 weeks.
DISCUSSION
Among various types of AIHA,4 warm antibody hemolytic anemia (WAHA), is the most common type of AIHA, where the immune system targets RBCs at normal body temperature (37°C). Antibodies, usually immunoglobulin G (IgG), attach to RBCs, leading to their destruction by macrophages in the spleen and liver.
Cold antibody hemolytic anemia (CAHA) is less common than WAHA. It involves the immune system attacking RBCs at lower temperatures and is typically triggered by exposure to cold. Cold agglutinins, often IgM antibodies, bind to RBCs in colder areas of the body, causing clumping and destruction.
Mixed-type antibody hemolytic anemia: Some individuals may exhibit characteristics of both WAHA and CAHA, making it a mixed-type presentation.
Drug-induced hemolytic anemia:5 Certain medications can trigger an immune response against RBCs, leading to hemolysis. Drugs can either induce the formation of antibodies that attack RBCs directly or cause a drug-induced immune reaction.
Secondary AIHA: AIHA can occur secondary to underlying conditions such as autoimmune diseases (e.g., lupus), infections or malignancies. Underlying diseases or conditions stimulate the immune system to produce antibodies that target RBCs.
IAIHA: In some cases, as described in this case, the exact cause of AIHA remains unknown, leading to the classification of IAIHA. The immune system appears to spontaneously target and destroy RBCs without any apparent triggering factor.
Management of AIHA involves addressing the underlying cause, if known, and utilizing immunosuppressive therapies to modulate the immune response and reduce destruction of the RBCs.
The idiopathic nature of IAIHA underscores the complexity of autoimmune disorders, highlighting the need for ongoing research to unravel the precise mechanisms triggering immune dysregulation in these cases. As our understanding of autoimmune diseases evolves, advancements in targeted therapies may offer more tailored and effective treatments for individuals with IAIHA.
CONCLUSION
This case highlights the importance of thorough investigation in new-onset anemia, particularly when standard treatments fail. It highlights the pitfalls of empiric treatments like iron, B12 and folic acid in outpatient settings for anemic patients. The successful management of IAIHA with corticosteroids showcases the significance of tailored therapies based on accurate diagnoses.
This case contributes to our understanding of IAIHA, emphasizing the need for continued research to advance treatment modalities. It also reinforces the critical role of comprehensive investigations in managing AIHA effectively.
Declaration
- Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
- Conflicts of interest/Competing interests: All authors declare that they have no conflicts of interest.
- Ethics approval: Ethical approval is not applicable for this article.
- Consent to participate: Written informed consent was obtained from the patient; the patient was informed of the potential risk-benefits and side effects of steroid treatment.
- Consent for publication: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
- Availability of data and material: All data underlying the results are available as part of the article and no additional source data are required.
- Code availability: Not applicable.
- Authors’ contributions: Drafting of the manuscript content, including medical writing of content – Dr Darpan Kothia and Analysis & interpretation of data - Dr Hemang K Acharya.
REFERENCES
- Petz LD, Garratty G. Immune Hemolytic Anemias. 2nd Edition. Churchill Livingstone; 2004.
- Hill QA, Stamps R, Massey E, Grainger JD, Provan D, Hill A; British Society for Haematology. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol. 2017;176(3):395-411.
- Michel M. Autoimmune hemolytic anemia: an approach to diagnosis and management. ASH Education Program Book. 2010;2010(1):69-73.
- Hill A, Hill QA. Autoimmune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2018;2018(1):382-9.
- Garratty G. Immune hemolytic anemia caused by drugs. Expert Opin Drug Saf. 2012;11(4):635-42.