Abstract
Background: In India, every year around 3.5 million babies are born premature, accounting for almost 13% of total live births in the country as compared to 5% to 7% incidence in the West. Preterm is defined as babies born before 37 weeks of pregnancy. The rapidity of feed volume increments involves controversies like faster weight gain, shorter hospital stays, the risk of necrotizing enterocolitis and vice versa. Methods: The present study was a randomized controlled trial conducted from June 1, 2018 to October 31, 2019. All infants in the study were randomized to slow and rapid feeding protocols by a stratified block randomization sequence of 2, 4, 6 blocks. Group 1 or the slow advancement group included 64 newborns babies and Group 2 or the rapid advancement group included 69 newborns babies. Results: The average weight gain in Group 1 was 4.41 ± 0.9 g and in Group 2 it was 6.33 ± 1.3 g, the difference was statistically significant (p < 0.02). Sixty out of 64 newborns regained birth weight within 16.87 ± 0.9 days in Group 1, while 64 out of 69 newborns regained birth weight within 13.63 ± 0.9 days in Group 2. The difference was statistically significant. Increment in the mean occipitofrontal circumference per week was 0.29 ± 0.27 cm Group 1, while in Group 2 it was 0.42 ± 0.05 cm; the difference was statistically significant. Mean average length increment per week was found to be 0.55 ± 0.04 cm and 0.69 ± 0.05 cm in Group 1 and Group 2, respectively, the difference was statistically significant (p < 0.005). The mean duration of hospital stay was 27.47 ± 3.33 days in Group 1 while in Group 2, the duration of stay was 23.15 ± 2.22 days, the difference was statistically significant. Conclusion: Our study supports enteral nutrition by rapid enteral feeding regimen in stable preterm neonates with very low birth weight.
Keywords: Very low birth weight, nutrition, feeding, enteral feeding, preterm
Globally, every year, an estimated 15 million babies are born preterm (before 37 completed weeks of gestation). Preterm birth complications are the leading cause of death among children under 5 years of age, responsible for approximately 1 million deaths in 2015.1
In India, every year almost 3.5 million babies are born premature, accounting for nearly 13% of total live births in the country as compared to 5% to 7% incidence in the West. Preterm birth is defined as babies born alive before 37 weeks of pregnancy are completed. There are subcategories of preterm births, based on gestational age.2 Complications of prematurity include necrotizing enterocolitis (NEC), feed intolerance, exaggerated physio-
logical jaundice, sepsis and prolonged hospital stay, etc. The duration of hospital stay may be reduced if feed can be rapidly built up thereby significantly reducing morbidity and mortality in these newborns. The appropriate goals of low birth weight feed include ensuring adequate short-term growth, preventing feeding-related morbidities, optimizing long-term outcomes including its impact on adult-onset diseases (e.g., coronary artery disease [CAD], diabetes mellitus, etc.). Over the last 2 decades, the concept of minimal enteral nutrition has evolved and is defined as starting a small amount of enteral feeding (exact volume not defined) usually 5-25 mL/kg as soon as possible after birth. This approach has numerous positive impacts on the development and maturation of gut function, hormonal and digestive enzyme surge.3,4 Numerous studies have shown the beneficial effects of minimal enteral nutrition; however, none have demonstrated an increase in the incidence of NEC. However, larger trials are required regarding safety in very immature and critically ill babies.5,6
The rapidity of increments in feed volume has been beset with controversies. A more rapid increase should result in faster weight gain and a shorter hospital stay. The proponents of slow feed advancements have cited the risk of NEC in their defense, while those in favor of rapid advancements have cited better growth in their defense. Controlled trials prior to the 1990s had observed an association between rapid feed advancement and increased risk of NEC.4,6 However, many randomized controlled trials have not demonstrated any increased risk of NEC.7-11 The lack of effect on NEC could be a result of differences in study design, improved neonatal care resulting in a decrease in NEC risk factors, and a shift in feeding protocols. The present study was aimed to compare the effects of slow rates of enteral feed advancement versus rapid advancement on the incidence of NEC in very low birth weight (VLBW) infants and to compare the effects of slow enteral feed advancement versus rapid advancement on the weight of VLBW infants. The primary outcome was the duration to reach full enteral feeds (days). The secondary outcomes were to study the incidence of feed intolerance, to find duration of hospital stay (days), to calculate average weight gain, length and head circumference and to find incidence of NEC.
MATERIALS AND METHODS
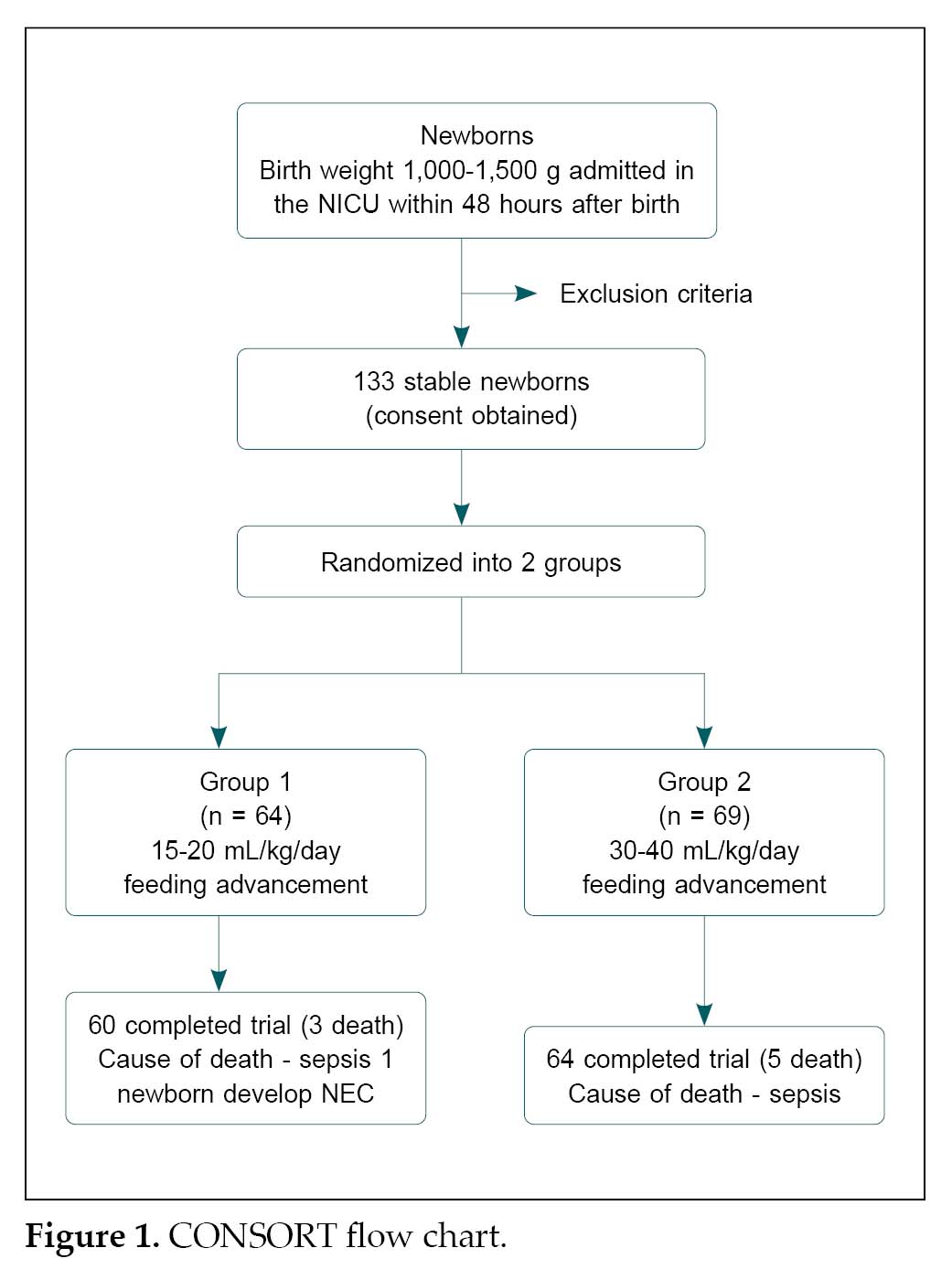
The present randomized controlled trial included 133 stable newborn babies, having a birth weight between 1,000 to 1,500 g, admitted in neonatal intensive care unit (NICU) in the Department of Pediatrics, SMS Medical College, and Attached Group of Hospitals, Jaipur during a period of 16 months from June 1, 2018 and October 31, 2019 after obtaining informed written consent of parents. Newborns with major congenital cardiac or other malformations contraindicating initiation of enteral feeds as per existing guidelines; with hypotension requiring dopamine ≥10 µg/kg/min or more than one inotrope support, persistent metabolic acidosis (pH <7.25 or base deficit of ≥10 mmol/L for >4 hours), abdominal distension, gastrointestinal bleeding and absent bowel sounds were excluded. All infants in the study were randomized into slow and rapid feeding protocols by a stratified block randomization sequence of 2, 4, 6 blocks. The slow advancement group (Group 1) comprised of 64 newborn babies and the rapid advancement group (Group 2) included 69 newborn babies. Both the study group newborns were thoroughly examined clinically on daily basis. The patients were monitored for weight gain, time to achievement of full feed, the occurrence of complications like NEC and sepsis. The findings and data were recorded on a predesigned proforma (Fig. 1).
The serial weight of the baby was recorded daily. Serial data of the length were recorded weekly. Abdominal girth was measured by using a nonstretchable tape, at the level, just above the umbilicus. Serial data of abdominal girth were recorded daily.
Feeding protocol: All neonates of both the groups were given gastrointestinal priming feeding (Trophic feeding) on Day 1 with expressed breast milk (EBM) at the rate of 10 mL/kg/day. All newborns were given feeding through a nasogastric tube.
Group 1 (Slow advancement group): All infants were given gastrointestinal priming feeding on Day 1 with EBM at the rate of 10 mL/kg/day, thereafter the feeds increased by 15-20 mL/kg/day till the maximum volume of 180 mL/kg/day was achieved.
Group 2 (Rapid advancement group): All infants in Group 2 were also given gastrointestinal priming as
in Group 1 infants. The feed was advanced by 30-40 mL/kg/day till the maximum volume of 180 mL/kg/day was achieved.
All stable VLBW neonates were thoroughly examined clinically daily and the finding were duly recorded on a predesigned proforma.
The pre-feed abdominal girth was measured. If pre-feed abdominal girth increased >2 cm between consecutive feeds, then gastric aspiration was done. If the pre-feed gastric aspirate volume was >30% or 3 mL/kg (whichever is greater) then the further increment of feed was deferred for the next 24 hours and one feed was omitted. If gastric aspirate volume was >50% of pre-feed, the feeding was discontinued temporarily for the next 24 hours.
During this period, the baby was investigated for evidence of sepsis and NEC. If sepsis was confirmed, the feed was discontinued and if sepsis screen was negative, feed was restarted at half the volume of last feed. In the event of the occurrence of severe sepsis or NEC, the patient was excluded from this study and managed for this complication.
RESULTS
In these VLBW babies, feed intolerance was observed clinically in relation to abdominal distension and gastric residue. We found that there was no statistically significant difference between the slow and rapid advancement groups. Both the time for reaching full-feed and duration of regaining birth weight were less in rapid advancement group than in slow advancement group. In the present study, total 133 VLBW babies were included.
In Group 1, 36 babies (56.25%) were male and 28 (43.75%) were female and 60 (93.75%) were appropriate-for-gestational age (AGA) and 4 (6.25%) were small-for-gestational age (SGA).
In Group 2, 43 (62.31%) were male and 26 (37.68%) were female and 65 (94.20%) were AGA and 4 (5.79%) were SGA.
The overall mean gestational age of newborns in Group 1 was 31.8 ± 0.3 weeks and that of Group 2 newborns was 31.9 ± 0.3 weeks. It was 31.63 ± 0.32 and 32.8 ± 2.04 weeks in Group 1 AGA and SGA newborns, while it was 31.89 ± 0.29 and 32.8 ± 1.36 weeks in Group 2 AGA and SGA newborns.
The mean birth weight of Group 1 newborns was 1,270.06 ± 38.2 g and those in Group 2 was 1,293.1 ± 36.2 g. It was 1,269.58 ± 38.58 and 1,299.69 ± 36.39 g in Group 1 AGA and SGA newborns, while it was 1,299.69 ± 36.39 and 1,263 ± 245.5 g in Group 2 AGA and SGA newborns (Table 1).
The mean time of starting minimal enteral feed in Group 1 was 25.6 ± 3.9 hours and 21.7 ± 3.8 hours in Group 2. It was 28.15 ± 5.42 and 27.0 ± 31.4 hours in Group 1 AGA and SGA newborns; while in Group 2 it was 21.35 ± 3.98 and 22.8 ± 21.12 hours in AGA and SGA newborns.
The mean time to achieve full feeds was 11.37 ± 1.36 days in Group 1, while in Group 2 was 6.59 ± 0.62 days. It was 10.75 ± 1.55 and 12.4 ± 8.76 days in Group 1 AGA and SGA newborns, while in Group 2 it was 6.63 ± 0.66 and 7.2 ± 3.44 days in AGA and SGA newborns. Feeding was interrupted in about 4.68% newborns in Group 1 and in 5.79% in Group 2.
The mean time of regaining birth weight was 16.87 ± 0.9 days in Group 1, while it was 13.63 ± 0.9 days in Group 2 (Table 2). It was 13.87 ± 1.88 and 16.0 ± 1.96 days in Group 1 AGA and SGA newborns, while in Group 2 it was 13.03 ± 1.16 and 11.0 ± 8.0 days in AGA and SGA newborns.
The average weight gain in Group 1 was 4.41 ± 0.9 g and 6.33 ± 1.3 g in Group 2 (Table 2). It was 3.61 ± 0.86 and 2.175 ± 2.09 g in Group 1 AGA and SGA newborns, while it was 5.66 ± 1.29 and 4.04 ± 6.22 g in AGA and SGA newborns in Group 2.
The mean occipitofrontal circumference increment per week was 0.29 ± 0.27 cm Group 1, while in Group 2, it was 0.42 ± 0.05 cm (Table 2). It was 0.28 ± 0.03 and 0.32 ± 0.14 cm in Group 1 AGA and SGA newborns,
|
Table 1. Socio-demographic Profile
|
|
Variable
|
Group 1
(n = 64)
|
Group 2
(n = 69)
|
P value
|
|
Male:Female
|
36:28
|
43:26
|
>0.05
|
|
Birth weight
(in grams)
|
1,270.06 ± 38.2
|
1,293.1 ± 36.2
|
0.39
|
|
Gestational age (In weeks)
|
31.8 ± 0.3
|
31.9 ± 0.3
|
0.42
|
|
AGA:SGA
|
60:4
|
65:4
|
>0.05
|
|
Table 2. Outcome Measures in Both Groups
|
|
Outcome
|
Group 1
(n = 64)
|
Group 2
(n = 69)
|
P value
|
|
Average weight gain per day (grams)
|
4.41 ± 0.9
|
6.33 ± 1.3
|
0.02
|
|
Regain of birth weight (days)
|
16.87 ± 0.9
|
13.63 ± 0.9
|
0.005
|
|
Average occipitofrontal circumference increment per week (cm)
|
0.29 ± 0.27
|
0.42 ± 0.05
|
<0.005
|
|
Average length increment per week (cm)
|
0.55 ± 0.04
|
0.69 ± 0.05
|
<0.005
|
|
Duration of hospital stay (Days)
|
27.47 ± 3.33
|
23.15 ± 2.22
|
0.03
|
while in Group 2 AGA and SGA newborns, it was 0.42 ± 0.06 and 0.32 ± 0.18 cm, respectively.
The mean length increment per week was found to be 0.55 ± 0.04 cm and 0.69 ± 0.05 cm in Group 1 and Group 2, respectively (Table 2). It was 0.53 ± 0.05 and 0.56 ± 0.03 cm in Group 1 AGA and SGA newborns, while it was 0.70 ± 0.05 and 0.54 ± 0.28 cm in Group 2 AGA and SGA newborns.
The mean duration of phototherapy was 92.04 ± 9.22 hours in Group 1 and it was 77.42 ± 7.54 hours in Group 2. It was 67.7 ± 12.8 and 67.2 ± 48.97 hours, respectively in Group 1 AGA and SGA newborns, while in Group 2 it was 62.28 ± 9.6 in AGA and 76.8 ± 64.6 hours SGA newborns.
In Group 1, NEC was found in 1 baby, while none of the newborns in Group 2 suffered from NEC.
In Group 1, sepsis was found in 3 babies, while it was seen in 5 babies in Group 2.
The mean duration of hospital stay was 27.47 ± 3.33 days in Group 1 while in Group 2, the duration of stay was 23.15 ± 2.22 days (Table 2). It was 27.9 ± 3.5 and 25.2 ± 14.86 days in Group 1 AGA and SGA newborns, while in Group 2 it was 23.37 ± 2.31 and 19.4 ± 9.48 days in AGA and SGA newborns.
Four newborns in Group 1 had adverse outcomes (expired/NEC), while 5 newborns in Group 2 had adverse outcomes (expired).
DISCUSSION
In the present study, study subjects included 64 newborns in Group 1 and 69 newborns in Group 2. The profile of patients was almost similar to that seen in other studies.7-13 In the present study, 93.75% newborns were AGA and 6.25% newborns were SGA in Group 1, while 94.2% were AGA and 5.80% newborns were SGA. In a study conducted by Krishnamurthy et al, in 80% newborns in Group 1 were AGA and 20% were SGA, while in Group 2, 72% newborns were AGA and 28% newborns were SGA.14 In a study conducted by Karagol et al, 65.21% newborns were AGA and 34.79% newborns were SGA in Group 1, while 63.04% newborns were AGA and 36.96% newborns were SGA in Group 2.8
In a study conducted by Krishnamurthy et al, the mean gestational age in Group 1 was 31.1 ± 1.2 weeks and 30.8 ± 1.1 weeks in Group 2.14 While in the study conducted by Karagol et al, the mean gestational age in Group 1 was 28.2 ± 1.1 weeks and in Group 2, it was 28.3 ± 1 weeks.8
In the present study, mean birth weight in Group 1 was 1,270.06 ± 38.2 g and in Group 2 was 1,293.1 ± 36.2 g. It was 1,269.58 ± 38.58 and 1,268 ± 250.6 g in Group 1 AGA and SGA newborns, while it was 1,299.69 ±
36.39 and 1263 ± 245.5 g in Group 2 AGA and SGA newborns. While in study conducted by Karagol et al, mean birth weight was in Group 1 was 984.3 ± 217.1 g
and in Group 2 was 951.6 ± 196.4 g. The difference was because Karagol et al had selected newborns with weight ranging from 750 to 1,250 g.8 The birth weight in the study conducted by Caple et al in 2004 was 1,000 to 2,000 g and <1,250 g in the study by Salhotra and Ramji in 2004 Krishnamurthy et al in 2010 reported a mean birth weight of 1,306.0 ± 129.2 g and 1,261.4 ± 121.6 g in Groups 1 and 2, respectively, which was similar to our study.7,13,14
In the present study, the mean time of starting minimal enteral feed in Group 1 was 25.6 ± 3.9 hours and 21.7 ± 3.8 hours in Group 2. It was 28.15 ± 5.42 and 27.0 ± 31.4 hours in Group 1 AGA and SGA newborns whiles in Group 2 it was 21.35 ± 3.98 and 22.8 ± 21.12 hours in AGA and SGA newborns. In Karagol et al, the mean time of starting minimal enteral feed was 36.4 (8-17) hours in Group 1 and 35.8 (11-22) hours in Group 2.8 In our study, trophic feeding in almost all babies were started within 24 hours, while in Karagol et al, the average time of starting trophic feeds was 24 to 48 hours.
In present study, the volume increments in Group 1 ranged from 1,520 mL/kg/day, while in Group 2, the increment in volume was 30-40 mL/kg/day. In Karagol et al, the volume increment in Group 1 was 20 mL/kg/day and 30 mL/kg/day in Group 2.8 In Rayyis (1999),12 the volume increment was 15 mL/kg/day in Group 1 and 35 mL/kg/day in Group 2. It was 20 mL/kg/day (Group 1) and 35 mL/kg/day (Group 2) in Caple et al (2004);13 15 mL/kg/day (Group 1) and 30 mL/kg/day (Group 2) in Salhotra and Ramji in 20047 and 20 mL/kg/day (Group 1) and 30 mL/kg/day (Group 2) in the study by Krishnamurthy et al.14
In the present study in Group 1 were given gastrointestinal priming feeding for first 24 hours with EBM at the rate of 10 mL/kg/day 4-hourly thereafter the feeds increased by 15-20 mL/kg/day till maximum achievement that was 180 mL/kg/day and similarly all the infants of Group 2 were also given gastrointestinal priming as Group 1 infants and feeds were advanced by 30-40 mL/kg/day till maximum of 180 mL/kg/day
was achieved. Salhotra and Ramji in 2004,7 and Krishnamurthy et al in 201014 and Karagol et al in 20138 also used the same maximum advancement of 180 mL/kg/day.
The method of feeding in the present study was nasogastric tube feeding similar to the other trials.9,11,12,14
Caple et al in 200413 used full strength commercial formula or human milk for feeding infants, Salhotra and Ramji in 20047 used human milk for feeding infants; Krishnamurthy et al14 used EBM and formula milk, while Karagol et al8 used EBM and formula milk for feeding. In the present study, we used EBM for the feeding of all infants.
In the present study, feeding was interrupted in 6.25% newborns in Group 1, whereas in Group 2, feeding was interrupted in 5.79%, newborns, which was statistically not significant. In Salhotra and Ramji in 2004,7 feeding was interrupted in 65.38% in Group 1 and in 51.62% in Group 2; in Krishnamurthy et al in 2010,14 feeding was interrupted in 24% in Group 1 and 16% in Group 2 newborns, while in Karagol et al in 2013,8 feeding had to be interrupted in 28% newborns in Group 1 and 23.9% in Group 2.
In the present study, no association was found between advancement of feed and feed intolerance. Salhotra and Ramji in (2004),7 Caple et al (2004)13 and Krishnamurthy et al (2010)14 also reported no significant difference in feed intolerance with slow and rapid advancements of feeds. It further strengthened the belief that adverse events related to rapid advancement of feeding were not so common as previously feared.
In present study, the fast advancement group reached full enteral feeds significantly earlier (mean 6.59 ± 0.62 days) than in the slow advancement group (mean 11.37 ± 1.36 days), which was statistically highly significant. It was 10.75 ± 1.55 and 12.4 ± 8.76 days in AGA and SGA newborns in Group 1, while it was 6.63 ± 0.66 and 7.2 ± 3.44 days in Group 2 AGA and SGA newborns. In Karagol et al, the mean duration to reach full enteral feed in Group 1 was 18.1 ± 5.8 days and 15.5 ± 8.4 days in Group 2.8 In Salhotra and Ramji in 2004, the fast advancement group (30 mL/kg/day) attained full enteral feeds of 180 mL/kg/day by the 10th day and the slow advancement group (15 mL/kg/day) by the 15th day (mean 14.8).7 In Rayyis et al, the rapid advancement group (35 mL/kg/day) attained full enteral feeds of 180 mL/kg/day by the 11th day and the slow advancement group (15 mL/kg/day) by the 15th day.12 In Krishnamurthy et al, the median time taken to reach full enteral feed was 9 days in Group 1 and 7 days in Group 2.14 But in the present study, the rapid advancement group (30-40 mL/kg/day) reached full feed by the 6.59 days (mean), while slow advancement group (15-20 mL/kg/day) reached full feed by 11.37 days (mean). Our results were similar to the studies by Salhotra and Ramji in 20047 and Krishnamurthy et al in 201014 in reaching full feeds early by rapid advancement of feeds without complications.
In the present study, the mean time to regain birth weight was shorter in the rapid advancement group (13.63 ± 0.9 days) compared to the slow advancement group (16.87 ± 0.9 days); this difference was statistically significant (p < 0.005). It was 13.87 ± 1.88 and 16.0 ± 1.96 days in Group 1 AGA and SGA newborns while in Group 2 it was 13.03 ± 1.16 and 11.0 ± 8.0 days in AGA and SGA newborns.
Salhotra and Ramji in 20047 also showed early regain of birth weight in slow advancement feeding group in 23 days, while in rapid advancement group, regain in birth weight occurred in 18 days. In the study conducted by Krishnamurthy et al, median time to regain birth weight was 22 days in Group 1 while it was 16 days in Group2.14 This study also supports our results of early regain of birth weight. Similar results were observed by Karagol et al.8 In which, mean time of regaining birth weight was in Group 1 19.3 (14-17) days and in Group 2 was 16.0 (12.3-20) days.
In our study, the average increment in length per week was 0.55 ± 0.04 cm in Group 1 and 0.69 ± 0.05 cm in Group 2. It was 0.53 ± 0.05 and 0.56 ± 0.03 cm, respectively in Group 1 AGA and SGA newborns, while it was 0.70 ± 0.05 and 0.54 ± 0.28 cm in Group 2 AGA and SGA newborns.
The mean duration of phototherapy was 92.04 ± 9.22 hours in Group 1, and 77.42 ± 7.54 hours in Group 2. This difference was due to slow enterohepatic circulation in slow advancement group, and in Group 1 AGA and SGA newborns, the mean duration of phototherapy was 67.7 ± 12.8 and 67.2 ± 48.97 hours, while in Group 2 AGA and SGA newborns, it was 62.28 ± 9.6 and 76.8 ± 64.6 hours.
In the present study, mean increment in occipitofrontal circumference per week was 0.29 ± 0.27 cm in Group 1, while in Group 2 it was 0.42 ± 0.05. It was 0.28 ± 0.03 and 0.32 ± 0.14 cm in Group 1 AGA and SGA newborns, and in Group 2 AGA and SGA newborns, it was 0.42 ± 0.06 and 0.32 ± 0.18 cm.
In present study, average weight gain per day was 4.41 ± 0.9 g in Group 1 and 6.33 ± 1.3 g, respectively. According to gestational age, in Group 1 it was 3.61 ± 0.86 and 2.17 ± 2.09 g in AGA and SGA newborns, while it was 5.66 ± 1.29 and 4.04 ± 6.22 g in Group 2 AGA and SGA newborns.
In the present study, the incidence of culture proven sepsis in Group 1 was 3.12% and 4.34% in Group 2.
In Salhotra and Ramji 2004,7 38.46% newborns in Group 1 and 18.51% in Group 2 developed sepsis. In Krishnamurthy et al in 2010,14 sepsis was seen in 10% and 8% of Group 1 and Group 2 patients, respectively. In Karagol et al (2013)8 sepsis was seen in 13.04% in Group 1 and 6.52% newborns in Group 2, respectively.
In the present study in Group 1 (slow advancement group), 1 male AGA newborn developed NEC. None of the newborns in the rapid advancement group developed NEC. Earlier studies have shown almost equal incidence of NEC in male and female newborns. In Caple et al in 2004,13 2.38% newborns in Group 1 and 5.40% in Group 2 developed NEC. In Salhotra and Ramji (2004),7 7.40% newborns in Group 2 developed NEC compared to none in Group 1. The incidence of NEC in Krishnamurthy in 201014 was 2% in Group 1 and 4% in Group 2, which showed similar ratio of NEC patients as present study. In Karagol et al,8 10.86% newborns in Group 1 and 8.69% newborns in Group 2 developed NEC. These studies show that is not a common adverse event in relation to feed advancement.
In the present study, mean duration of hospital stay was 27.47 ± 3.3 days in Group 1 and 23.15 ± 2.2 days in Group 2; it was 27.9 ± 3.5 and 25.2 ± 14.86 in Group 1
AGA and SGA newborns, and 23.37 ± 2.31 and 19.4 ± 9.48 in Group 2 AGA and SGA newborns. In Karagol et al,8 the mean duration of hospital stay was 26.2 ± 1.1 days in Group 1 and 21.5 ± 1.3 days in Group 2.
The adverse events related to rapid advancement of feeding, i.e., gastric residuals, abdominal distension and feeding interruption were comparable in the slow advancement and rapid advancement group. The benefits of rapid advancement of feeds were early regaining of birth weight, shorter hospital stay and early achievement of full feed. Similar conclusion was also found in earlier studies by Salhotra and Ramji in 20047 and Caple et al in 200413 and Krishnamurthy et al 2010.14
Feed intolerance (abdominal distension, gastric residue, feed interruption) related to advancement of feeding were almost equal in slow advancement and rapid advancement group indicating that rapid advancement of feeding is beneficial in VLBW babies and refutes the old fear about the rapid advancement of feeding in low birth weight and premature babies.
CONCLUSIONS
In conclusion, rapid advancement of feeding up to 40 mL/kg/day is safe and well-tolerated in VLBW babies, including SGA and AGA. It leads to early regaining of birth weight and reduces the total duration of hospital stay of newborns, which can indirectly decrease the risk of hospital-acquired infections and other complications. It fulfills the required nutrition and prevents undernutrition and future growth retardation of VLBW babies.
It lessens the physical and psychological stress of parents and reduces the extra work load of the medical personnel and hospitals and also cuts short the financial burden on hospitals, families and heath personnel by shortening hospital stay in developing countries like India. But further long-term studies are required with large numbers of premature and low birth weight babies, to recommend universal rapid advancement of feeding in these babies.
Declarations
Funding: None of the authors has taken any financial support from anyone.
Conflicts of interest/Competing interests: None.
Availability of data and material: Yes.
Code availability: Not applicable.
Authors’ contributions: RKM & DRB drafted the study design, collected data, contributed in statistical analysis and inference of results. RM & JS supervised the entire work, drafted the study design, collected data, contributed in statistical analysis and inference of results. CKM did critical analysis of all data, counseled the parents and hypothesized the study, drafted the study design, collected data, contributed in statistical analysis and inference of results. RKM, DRB, JS, CM & RM drafted the conclusions, reviewed the literature, drafted the study design, collected data, contributed in statistical analysis and inference of results.
Ethics approval: Taken.
Consent to participate: Consent of parents was taken.
Consent for publication: Yes.
REFERENCES
- Cloherty and Stark's Manual of Neonatal Care-South Asian. 8th Edition. Wolters Kluwer; 2017. pp. 79-161.
- Singh M. Care of the Newborn. CBS Publishers and Distributors Pvt. Ltd., Revised 8th Edition, 2017. p. 301.
- Gleason CA, Devaskar SU. Avery's Diseases of the Newborn Book. 9th Edition, 2012. pp. 399-499.
- Cloherty and Stark's Manual of Neonatal Care-South Asian. 8th Edition. Wolters Kluwer; 2017. pp. 80-2.
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388
(10063):3027-35.
- Poindexter BB, Langer JC, Dusick AM, Ehrenkranz RA; National Institute of Child Health and Human Development Neonatal Research Network. Early provision of parenteral amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. J Pediatr. 2006;148(3):300-5.
- Salhotra A, Ramji S. Slow versus fast enteral feed advancement in very low birth weight infants: a randomized control trial. Indian Pediatr. 2004;41(5):435-41.
- Karagol BS, Zenciroglu A, Okumus N, Polin RA. Randomized controlled trial of slow vs rapid enteral feeding advancements on the clinical outcomes of preterm infants with birth weight 750-1250 g. JPEN J Parenter Enteral Nutr. 2013;37(2):223-8.
- Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):
1253-61.
- McGuire W, Bombell S. Slow advancement of enteral feed volumes to prevent necrotizing enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2008;2:CD001241.
- Anderson DM, Kliegman RM. The relationship of neonatal alimentation practices to the occurrence of endemic necrotizing enterocolitis. Am J Perinatal. 1991;8(1):62-7.
- Rayyis SF, Ambalavanan N, Wright L, Carlo WA. Randomized trial of “slow” versus “fast” feed advancements on the incidence of necrotizing enterocolitis in very low birth weight infants. J Pediatr. 1999;134(3):293-7.
- Caple J, Armentrout D, Huseby V, Halbardier B, Garcia J, Sparks JW, et al. Randomized, controlled trial of slow versus rapid feeding volume advancement in preterm infants. Pediatrics. 2004;114(6):1597-600.
- Krishnamurthy S, Gupta P, Debnath S, Gomber S. Slow versus rapid enteral feeding advancement in preterm newborn infants 1000-1499g: a randomized controlled trial. Acta Paediatr. 2010;99(1):42-6.