Published in IJCP
August 2024
Review Article
Iron Deficiency in Heart Failure: Unveiling the Hidden Culprit
August 09, 2024 | Kamal Kishor, Ashwani Kumar, Devendra Singh Bisht, Shekha Vohra
Cardiology
Iron deficiency (ID) is a frequent comorbidity in patients with heart failure (HF). Coexistent HF and ID make the issues more challenging to diagnose and treat. Iron deficiency exacerbates clinical symptoms, impairs quality of life and increases the risk of recurrent hospitalization for HF. Conversely, a proinflammatory state and altered gut kinetic in HF may result in absolute or functional ID, which conventional laboratory makers may not diagnose and differentiate accurately. Novel diagnostic markers like soluble transferrin receptor (sTfR), reticulocyte hemoglobin concentration, red blood cell distribution width, sTfR: log (ferritin) ratio and serum hepcidin levels may help to diagnose ID more accurately in the setting of HF. The intravenous (IV) iron formulation has shown promising results in improving the functional class and reducing recurrent hospitalization in patients with HF and ID. Futuristic therapies like nanosized iron preparations, hepcidin inhibitors and hepcidin antagonists may help manage ID more efficiently and conveniently in HF. This manuscript explores the relationship between ID and HF. It also provides the latest information related to the diagnosis and treatment of ID in HF patients.
Keywords: Iron deficiency, heart failure, intravenous iron formulation
Iron is essential to executing several physiological processes, such as oxygen transport and storage, cellular respiration, energy metabolism, and control of vascular tone1. Like all other chronic inflammatory diseases, iron deficiency (ID) frequently afflicts patients with heart failure (HF). ID is a common comorbidity in patients with HF, affecting up to 50% of all ambulatory patients2. Prevalence varies depending on the criteria used to diagnose ID in the cohort analyzed3. ID is associated with worsening of the signs and symptoms of HF, poor quality of life, and increased risk of recurrent hospitalization and death4. Strategies targeting ID in the HF cohort significantly improve clinical outcomes, New York Heart Association (NYHA) functional class, exercise capacity, and quality of life5-10.
Iron is vital for the human body. In free form, iron causes oxidative injury and may lead to premature cell death. Iron, therefore, must remain bound to protein to ensure safety and stability. Iron is stored as an iron-protein complex in the liver, spleen, and reticuloendothelial cells (storage pool). In the bloodstream, it is transported conjugated with apo-transferrin as transferrin. Transferrin carries iron around the body, which is physiologically usable by cells after uptake by transferrin receptor-1 (functional pool). Three key regulators are crucial in iron homeostasis. Ferroportin regulates iron absorption from the gut and its discharge from the stored pool. Hepcidin induces internalization and degradation of ferroportin and acts as a master regulator of iron metabolism. Lastly, erythroferrone is a negative regulator of iron metabolism. It inhibits hepcidin production, thus ensuring iron availability for erythropoiesis11-13. All these proteins work in a coordinated manner to maintain a stable level of iron in the body.
EFFECT OF HF ON IRON HOMEOSTASIS
Heart failure is a proinflammatory condition. It is associated with elevated levels of inflammatory cytokines, which result in elevated hepcidin levels. Hepcidin negatively interacts with ferroportin, hindering iron absorption from the gut and its release from the storage pool. In this way, patients with HF are at risk of both absolute (depleted iron stores) and functional ID (sufficient iron not available at the cellular level despite normal total body iron)13. Within the framework of HF, many other factors may precipitate the state of ID (Table 1). Some of these factors include nutritional deficiency, poor bioavailability of dietary iron, edematous gut, blood loss secondary to antiplatelet or anticoagulant medication and associated comorbidities2.
|
Table 1. Fundamental Causes of Iron Deficiency in Heart Failure
|
|
Poor absorption due to increased gut thickness
|
|
Poor absorption due to gut edema
|
|
Poor bioavailability of dietary iron
|
|
Blood loss due to antiplatelets or anticoagulants
|
|
Associated comorbidities
|
|
Elevated hepcidin levels
|
|
Elevated inflammatory cytokines
|
EFFECT OF IRON DEFICIENCY ON HF OUTCOMES
Iron is crucial for synthesizing hemoglobin, myoglobin, cytochrome c, and many other proteins. It acts as a cofactor of several enzymes, including nitric oxide synthase. ID, therefore, has a deleterious effect on oxygen transportation (hemoglobin) and aerobic respiration of muscles (myoglobin), oxidative phosphorylation (cytochrome c), and other processes dependent on a sufficient level of iron (Table 2)1,13. Patients with HF and ID exhibit a high prevalence of anemia, adverse remodeling, and worse muscular symptoms (such as muscle pain, fatigue, cramps, shortness of breath, and poor exercise capacity) secondary to insufficient heme production. ID disturbs oxidative phosphorylation and reduces adenosine triphosphate (ATP) production. Therefore, tissues with higher energy requirements (such as the myocardium, skeletal muscle, and the central nervous system) are affected most by ID. Lastly, ID disrupts the production of nitric oxide synthase (NOS), resulting in vasoconstriction. This, in turn, worsens symptoms like difficulty breathing and fatigue, which also result in recurrent hospitalization1,13.
|
Table 2. Critical Substrates Affected by Iron Deficiency1,13
|
|
Substrate
|
Essential role
|
Impact of ID on patients with HF
|
|
Hemoglobin
|
Oxygen transportation
|
Anemia, adverse remodeling of the left ventricle, fatigue, shortness of breath, recurrent hospitalization, increased cardiovascular death.
|
|
Myoglobin
|
Oxygen storage in skeletal muscle
|
Muscle pain, fatigue, cramps, shortness of breath, and poor exercise capacity.
|
|
Cytochrome c
|
Electron transport chain for ATP generation
|
Adverse impact on tissues with higher energy requirements such as the myocardium, skeletal muscle, and the central nervous system.
|
|
Nitric oxide synthase
|
Control of vascular tone
|
Worsens symptoms like difficulty breathing, fatigue, and recurrent hospitalization.
|
DEFINING IRON DEFICIENCY IN HEART FAILURE
Iron deficiency in HF can be absolute iron deficiency (AID) or functional iron deficiency (FID). In AID iron, the total body stores of iron get depleted. Conversely, FID typifies a situation where iron stores are normal or elevated (Table 3)14-16. However, the downregulation of ferroportin and upregulation of hepcidin and other inflammatory cytokines hinder iron mobilization from the storage pool. Ferritin levels (reflection of storage pool) and transferrin saturation (reflection of iron bioavailability) are the two most commonly used laboratory parameters to define ID in routine clinical settings (Fig. 1). Serum ferritin <30 µg/L and transferrin saturation (TSAT) <16% suggest a state of ID14. However, considering the effect of HF on serum ferritin and TSAT, it is imperative to change the criterion of diagnosing ID in the setting of HF. Serum ferritin is an acute phase reactant and gets nonspecifically elevated in conditions with activated inflammatory pathways such as HF. This change correlates with the extent of inflammation. Conversely, transferrin is a negative phase reactant and may get suppressed in HF15.
|
Table 3. Absolute and Functional Iron Deficiency in Heart Failure14-16
|
|
Absolute iron deficiency
- Depletion of both storage and functional pool of iron.
- Diagnosed as serum ferritin (a marker of storage pool) <100 μg/L and transferrin saturation (a marker of the functional pool) <20%.
|
|
Functional iron deficiency
- Normal or elevated storage pool but sequestration of the functional pool of iron.
- Diagnosed as serum ferritin (a marker of storage pool) 100-300 μg/L and transferrin saturation (a marker of the functional pool) <20%.
|
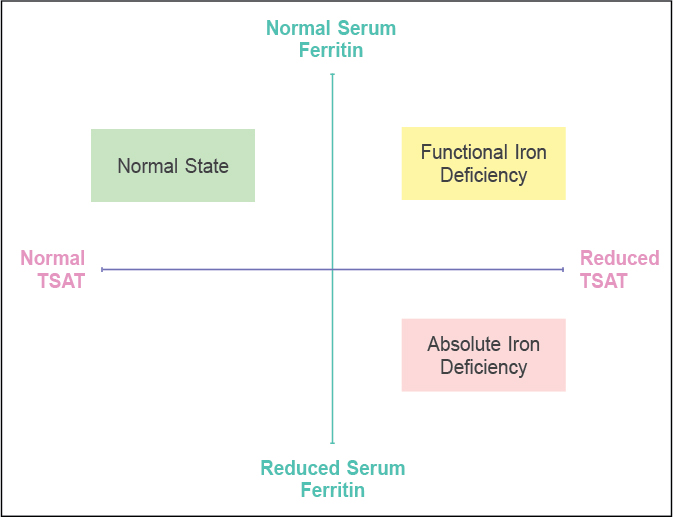
Figure 1. Absolute and functional iron deficiency in patients of heart failure. In AID, both iron storage and functional pools are depleted. However, in FID, the storage pool remains normal or even elevated, but the iron cannot move out of the storage pool. This may be due to the impact of the altered hepcidin-ferroportin axis in HF. This leads to improper iron availability to erythroid and nonerythroid tissue despite a regular storage pool.
Although there is no precise serum ferritin and TSAT cut-off, serum ferritin levels <100 µg/L or 100-300 µg/L if TSAT is <20% diagnose ID in HF with 82% sensitivity and 72% specificity16. Novel markers such as soluble transferrin receptor (sTfR), reticulocyte hemoglobin concentration, and red blood cell distribution width (RDW) can diagnose ID more accurately in patients with HF. However, these markers have yet to be routinely used. Additionally, measuring sTfR, sTfR: log (ferritin) ratio or hepcidin levels could be more effective in discriminating AID from FID17. A detailed description of all these investigations is beyond the scope of this manuscript.
MANAGEMENT OF IRON DEFICIENCY IN HEART FAILURE
Review of Literature
Clinical trials and meta-analyses have suggested beneficial effect of IV iron in patients with ID (Table 4)5-10,18-20. In the FAIR-HF (Ferinject Assessment in patient with IRon deficiency and chronic Heart Failure) trial, the use of IV ferric carboxymaltose in patients of HF with ID showed significant improvement in NYHA classification, functional capacity, and quality of live5. The results were similar in patients with anemia and without anemia. AFFIRM-AHF, a randomized, double-blind, placebo-controlled trial comparing the effect of IV ferric carboxymaltose on hospitalizations and mortality in iron deficient subjects admitted for acute HF, investigated the effect of IV ferric carboxymaltose on outcomes in patients who were stabilized after an episode of acute HF. Patients who received IV ferric carboxymaltose, showed a significant reduction in HF hospitalization compared to placebo (relative risk [RR], 0.74; 95% confidence interval [CI], 0.58-0.94) but no reduction in cardiovascular death8. IRONMAN (Effectiveness of Intravenous Iron Treatment versus Standard Care in Patients with Heart Failure and Iron Deficiency) trial investigated the long-term safety and efficacy of IV ferric derisomaltose in a predominately outpatient HF population. Data suggested numerically lower HF hospitalizations and cardiovascular death in patients who received the ferric derisomaltose arm (22.4 per 100 patient-years) than in the placebo arm (27.5 per 100 patient-years). However, it failed to reach significance (p = 0.07)19.
|
Table 4. Conclusion of Major Clinical Trials and Meta-analyses Related to the Role of Intravenous Iron Therapy in Patients with Heart Failure5-10,18-20
|
|
Study
|
Year
|
Conclusion
|
|
FAIR-HF5
|
2009
|
- Treatment with intravenous (IV) ferric carboxymaltose improves symptoms, functional capacity and quality of life in patients with chronic HF and ID, regardless of anemia.
- IV ferric carboxymaltose has an acceptable side-effect profile.
|
|
CONFIRM-HF6
|
2015
|
- IV ferric carboxymaltose over 1 year in symptomatic iron deficient HF patients resulted in sustainable improvement in functional capacity, symptoms, and quality of life.
- It may be associated with a risk reduction of hospitalization for worsening HF.
|
|
IRONOUT-HF7
|
2017
|
- High-dose oral iron over 16 weeks did not improve exercise capacity among participants with HF with reduced ejection fraction (HFrEF) with ID.
|
|
AFFIRM-AHF8
|
2020
|
- Treatment with IV ferric carboxymaltose in patients with left ventricular ejection fraction (LVEF) of <50%, ID and who were stabilized after an episode of acute HF resulted in reduced risk of HF hospitalizations.
- There was no apparent effect on the risk of cardiovascular death.
|
|
IRONMAN19
|
2022
|
- In patients with HF, reduced LVEF, and ID, IV ferric derisomaltose administration was associated with a lower risk of hospital admissions for HF and cardiovascular death.
|
|
HEART-FID18
|
2023
|
- Ambulatory patients of HFrEF and ID, did not demonstrate any benefit in hierarchical composite of death, hospitalizations for HF, or 6-minute walk distance with IV ferric carboxymaltose.
|
|
Meta-analysis by Myint et al20
|
2022
|
- Patients with HF and ID demonstrated lower HF hospitalizations with IV iron therapy.
- IV iron therapy improves the quality of life and decreases health care expenditure.
|
|
Meta-analysis by Graham et al9
|
2023
|
- In HF patients with ID, IV iron therapy reduces the risk of hospitalization for HF.
- Its effect on cardiovascular or all-cause mortality remains inconclusive.
|
|
Meta-analysis by Salah et al10
|
2023
|
- IV iron therapy in patients with HF reduces the composite risk of first hospitalization for HF and cardiovascular mortality.
- There was a reduction in first and recurrent hospitalizations for HF.
- No effect on all-cause mortality or cardiovascular mortality.
|
A meta-analysis conducted by Myint et al revealed a 13.8% decreased risk of HF hospitalizations (odds ratio [OR] 0.59; 0.35-0.98, p = 0.040) and a 17.5% reduction in the composite outcome of HF hospitalizations or cardiovascular mortality. All-cause and cardiovascular mortality were not different between IV iron and placebo groups20.
Similar results were observed in a meta-analysis of 10 randomized controlled trials by Graham et al. It suggested a significant reduction in composite outcomes of recurrent hospitalizations for HF and cardiovascular mortality (rate ratio 0.75, 95% CI 0.61-0.93; p < 0.01) and first hospitalization for HF or cardiovascular death (OR 0.72, 95% CI 0.53-0.99; p = 0.04) in the group of patients who received IV iron as compared to those who received placebo or standard care. Similar to the prior meta-analysis, effects on cardiovascular (OR 0.86, 95% CI 0.70-1.05; p = 0.14) and all-cause mortality (OR 0.93, 95% CI 0.78-1.12; p = 0.47) were inconclusive9.
Calculating Iron Deficit
Conventionally, the total iron deficit is estimated using the modified Ganzoni formula21. This equation estimates iron deficit by calculating iron attached to deficit hemoglobin. In healthy individuals, blood volume is 7% (0.07) of body weight. Iron content in hemoglobin is 0.34% (0.0034) g/dL. So, the total iron deficit in mg/L will be as below.
Iron deficit = weight (kg) × (target Hb - actual Hb) × 0.0034*0.07*10,000 + Depot iron.
Depot iron here represents an extra depot dose to replenish iron stores. This is usually kept at 500 mg. Notably, the above-mentioned formula may not accurately reflect the actual iron needs of the patient. It is crucial to consider other factors, such as inflammation, comorbidities and medication while estimating iron deficits in HF patients.
Selection of Iron Formulation
Oral iron formulations are not the preferred choice to treat ID in patients with HF. The IRONOUT HF (Iron Repletion effects ON Oxygen UpTake in Heart Failure) trial investigated the effects of oral iron therapy on exercise capacity and quality of life score in patients with HF with reduced ejection fraction and ID. Despite improvement in iron biomarkers such as transferrin saturation and ferritin levels, exercise capacity was not improved compared to placebo7. Unlike oral iron therapy, IV iron therapy benefits the 6-minute walk test distance, peak oxygen consumption, quality of life, and improvement in NYHA class9. IV iron formulations contain a core of iron hydroxide enveloped by variable carbohydrate complexes22. The carbohydrate envelope in the earlier formulation was composed of high molecular weight dextran. These formulations are no longer preferred due to the high risk of serious drug events associated with them4. Iron sucrose, ferric carboxymaltose, and ferric derisomaltose are the three most commonly studied nondextran IV iron formulations to treat ID in patients with HF (Table 5)5,6,8,18,19,23,24. Data from a recent meta-analysis demonstrated no significant difference in outcome between different IV iron-carbohydrate formulations when similar end points were measured25.
|
Table 5. Intravenous Iron Formulation Available for Management of Iron Deficiency in Heart Failure5,6,8,18,19,23,24
|
|
Formulation
|
Maximum dose (in single setting)
|
Duration of administration
|
Evidence in HF
|
|
Iron sucrose
|
200 mg
|
30 min
|
Toblli et al23
FERRIC-HF24
|
|
Ferric carboxymaltose
|
1,000 mg
|
15 min
|
FAIR-HF5, AFFIRM-AHF8, HEART-FID18, CONFIRM-HF6
|
|
Ferric derisomaltose
|
20 mg/kg up to 2,000 mg
|
20 min
|
IRONMAN19
|
Current Guidelines
Based on ample evidence, the 2021 European Society for Cardiology guidelines and the 2022 AHA/ACC/HFSA guidelines recommend using IV iron to improve functional status and quality of life in groups of patients with HF and ID (Table 6)26,27.
|
Table 6. Clinical Guidelines for the Management of Iron Deficiency in Heart Failure
|
| |
European Society of Cardiology Guidelines
|
ACC/AHA Guideline
|
|
Recommendation of screening
|
All patients with HF (COR I; LOE C)
|
All patients with HF
|
|
Target population
|
Symptomatic patients with LVEF <45% (COR II; LOE A)
Patients recently hospitalized for HF with LVEF <50% to reduce risk of hospitalization (COR II; LOE B)
|
Patients with HFrEF and ID (COR II; LOE A)
|
|
Treatment effect
|
Symptomatic patients with LVEF <45%: Alleviate HF symptoms, improve exercise capacity and QOL (COR II; LOE A)
Symptomatic patients recently hospitalized for HF with LVEF <50%: Reduce the risk of hospitalization (COR II; LOE B)
|
Improve exercise capacity and QOL (COR II; LOE A)
|
|
Diagnosis
|
Serum ferritin <100 or
Serum ferritin 100-300, if TSAT <20%
|
Serum ferritin <100 or
Serum ferritin 100-300, if TSAT <20%
|
|
Management
|
Iron replacement with IV ferric carboxymaltose
|
IV iron replacement
|
Novel markers and therapeutic options for managing ID are in the research spotlight. Newer diagnostic markers diagnose ID more accurately in the HF setting and can differentiate AID from FID. Some key diagnostic markers are sTfR, reticulocyte hemoglobin concentration, RDW, sTfR: log (ferritin) ratio, and serum hepcidin levels. sTfR levels increase in response to ID to absorb more iron. sTfR, therefore, may diagnose functional iron status more accurately than traditional markers.
Reticulocyte hemoglobin concentration represents iron available for new red blood cell production. Low levels of reticulocyte hemoglobin concentration are a clear sign of ID. RDW measures the red blood cell size variation. Higher RDW values suggest disrupted red cell production, which could indicate ID. sTfR: log (ferritin) ratio is another marker that helps distinguish FID from AID. Higher levels are consistent with FID. Lastly, the estimation of hepcidin levels helps to understand iron homeostasis. Hepcidin inhibits iron’s release from body stores. Thus, elevated levels are consistent with FID17. Nanosized iron preparations are emerging oral iron therapies that may help to resolve concerns of poor absorption and gastrointestinal side effects related to traditional oral iron therapy28.
Compounds that antagonize hepcidin or its effects could help ameliorate HF-associated ID and are in the research phase. The use of such compounds in clinical settings warrants further investigation and testing29. While more research is needed to fully understand the safety and efficacy of these newer therapeutic options, they represent exciting advances in cardiovascular medicine.
Iron deficiency is a frequent comorbidity in HF patients. HF adversely affects iron homeostasis, leading to states ranging from AID to FID. According to current guidelines, ID in HF is diagnosed using serum ferritin and transferrin. However, upcoming markers such as sTfR, reticulocyte hemoglobin concentration and RDW may help diagnose ID more accurately in patients with HF. Further, novel investigations such as sTfR, sTfR: log (ferritin) ratio, or hepcidin levels could more effectively discriminate AID from FID. Once diagnosed, nondextran IV iron therapy is safe and effective in improving functional class and quality of life in patients of ID with HF.
Although current therapy does not offer cardiovascular mortality benefits, innovative therapies (hepcidin inhibitor, nanoparticle and gene therapy) may be more effective for treating ID.
|
Key Messages of the Current Manuscript
- Iron deficiency worsens the clinical symptoms and functional class related to HF.
- Patients with HF may suffer from absolute or functional ID. These two conditions may happen independently or simultaneously.
- Serum ferritin and transferrin saturation can diagnose ID with modest sensitivity and specificity in the HF setting. Novel markers for a more accurate diagnosis of ID and distinguishing the two types are in the research phase.
- Nondextran IV iron formulation therapy is currently approved for managing ID in HF. Newer therapies like nanosized iron preparation and therapy targeting hepcidin are under clinical trials.
|
Disclosure: No funding was received in the publication of this article.
Declarations of interest: None.
Declaration of Generative AI and AI-assisted Technologies in the Writing Process
During the preparation of this work the authors used Grammarly AI tool in order to improve readability and language. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
- Dunn LL, Suryo Rahmanto Y, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17(2):93-100.
- Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165(4):575-82.e3.
- Yeo TJ, Yeo PS, Ching-Chiew Wong R, Ong HY, Leong KT, Jaufeerally F, et al. Iron deficiency in a multi-ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance, and prognosis. Eur J Heart Fail. 2014;16(10):1125-32.
- Beavers CJ, Ambrosy AP, Butler J, Davidson BT, Gale SE, Mastoris I, et al. Iron deficiency in heart failure: a scientific statement from the Heart Failure Society of America. J Card Fail. 2023;29(7):1059-77.
- Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. New Engl J Med. 2009;361(25):2436-48.
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657-68.
- Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, et al; NHLBI Heart Failure Clinical Research Network. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA. 2017;317(19):1958-66.
- Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, et al; AFFIRM-AHF investigators. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020;396(10266):
1895-904.
- Graham FJ, Pellicori P, Kalra PR, Ford I, Bruzzese D, Cleland JGF. Intravenous iron in patients with heart failure and iron deficiency: an updated meta-analysis. Eur J Heart Fail. 2023;25(4):528-37.
- Salah HM, Savarese G, Rosano GMC, Ambrosy AP, Mentz RJ, Fudim M. Intravenous iron infusion in patients with heart failure: a systematic review and study-level meta-analysis. ESC Heart Fail. 2023;10(2):1473-80.
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101(7):2461-3.
- Franchini M, Montagnana M, Lippi G. Hepcidin and iron metabolism: from laboratory to clinical implications. Clin Chim Acta. 2010;411(21-22):1565-9.
- Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138(1):80-98.
- Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341(26):1986-95.
- Murphy SP, Kakkar R, McCarthy CP, Januzzi JL Jr. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(11):1324-40.
- Grote Beverborg N, Klip IT, Meijers WC, Voors AA, Vegter EL, van der Wal HH, et al. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail. 2018;11(2):e004519.
- Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1 Suppl 1:S4-8.
- Mentz RJ, Garg J, Rockhold FW, Butler J, De Pasquale CG, Ezekowitz JA, et al; HEART-FID Investigators. Ferric carboxymaltose in heart failure with iron deficiency. N Engl J Med. 2023;389(11):975-86.
- Kalra PR, Cleland JGF, Petrie MC, Thomson EA, Kalra PA, Squire IB, et al; IRONMAN Study Group. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): an investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. Lancet. 2022;400(10369):2199-209.
- Myint PT, Nandar PP, Thet AM, Orasanu G. Cost-effective heart failure management: Meta-analysis of IV iron therapy in iron-deficient heart failure patients. Am Heart J Plus Cardiol Res Pract. 2022;22:100204.
- Ganzoni AM. Intravenous iron-dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr. 1970;100(7):301-3.
- Von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail. 2019;7(1):36-46.
- Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50(17):1657-65.
- Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency: FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51(2):103-12.
- Sindone A, Doehner W, Comin-Colet J. Systematic review and meta-analysis of intravenous iron-carbohydrate complexes in HFrEF patients with iron deficiency. ESC Heart Fail. 2023;10(1):44-56.
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-726.
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263-421.
- Singh K, Chopra DS, Singh D, Singh N. Nano-formulations in treatment of iron deficiency anaemia: an overview. Clin Nutr ESPEN. 2022;52:12-9.
- Sagar P, Angmo S, Sandhir R, Rishi V, Yadav H, Singhal NK. Effect of hepcidin antagonists on anemia during inflammatory disorders. Pharmacol Ther. 2021;226:107877.
|