Introduction: Malnutrition is widely prevalent in
developing countries and is considered a common denominator in infant and
under-5 mortalities. Most child deaths are associated with inappropriate
feeding practices and specific micronutrient deficiencies during the first year
of life. There is a lack of data about severe acute malnutrition (SAM) and
specific micronutrient deficiencies in India; hence, the present study was
conducted to study the iron profile, including folic acid, and vitamin B12
levels and their correlation with the clinicoepidemiological profile of
children with SAM. Materials and methods: This hospital-based cross-sectional
study included 95 children with SAM, aged 6 months to 5 years.
A predesigned structured proforma was used to collect information. Data
concerning clinical examination and history given by the mother and a reliable
attendant was collected. The quantitative data were expressed as mean and
standard deviation and qualitative data as percentage and proportion. The
difference in proportion was analyzed by the Chi-square test and the difference
in means was analyzed by ANOVA. P-value <0.05 was taken as significant. All
calculations were done by Microsoft Excel, Primer. SPSS Software [version 21]
was used for doing statistical analysis. Results: In the present study,
a total of 95 SAM patients were included with mean age 19.74 months and an F:M
ratio of 1.2:1. Weight-for-height was found to be the most reliable criterion
to identify children with SAM (78.95%). Edema was present in 18 (18.95%)
patients. Around 68.42% of patients had mid-upper arm circumference (MUAC)
<11.5 cm; 25.26% of children were found completely immunized, remaining
74.74% were either partially immunized or unimmunized. According to the
Kuppuswamy scale for the socioeconomic class, more than two-thirds of the
parents belonged to the upper-lower class. About 44.21% of children received
exclusive breastfeeding till 6 months of age, while complementary feeding was
started in only 25.26% of children at 6 months of age. Anemia was present in 93
children with a prevalence of 97.89%. Of these, 30 patients had vitamin B12
deficiency anemia, 20 patients had iron deficiency anemia, and 6 patients had
folate deficiency anemia. Conclusions: Severe acute malnutrition is an
important preventable and treatable cause of morbidity and mortality in
children below 5 years of age in India. Although malnutrition is highly
prevalent in Indian children, there are very limited data that use biochemical
indexes to characterize the epidemiology of micronutrient deficiencies in
children with SAM. A detailed understanding of micronutrient deficiencies and
clinical and epidemiological profile of children may help in micronutrient
supplementation and fortification programs and targeting the basic causes of
pediatric mortalities.
Keywords: Severe acute malnutrition, NFHS-5, iron, folic acid, vitamin B12,
wasting, stunting
Childhood undernutrition is an underlying
cause of an estimated 45% of all deaths among under-5 children1.
India has an under-5 mortality rate (U5MR) of 41.9 per 1,000 live births.
According to the National Family Health Survey (NFHS-5) 2019-21, 35.5% of children
under 5 years are stunted (Below –2 standard deviations [SD], based on the
World Health Organization [WHO] standard, height-for-age); 19.3% of children
under 5 years are wasted (< –2SD, WHO standard, weight-for-height), 7.7% of
children under 5 years are severely wasted (< –3SD, WHO standard;
weight-for-height), 32.1% of children under 5 years are underweight (< –2SD,
based on the WHO standard, weight-for-age); and 3.4% of children under 5 years
are overweight (> +2SD, based on the WHO standard, weight-for-height)2.
It is estimated that 1 in every 3 malnourished
children live in India. Malnutrition in children is widely prevalent in
developing countries and has been responsible for 60% of the 10.9 million
deaths annually among children less than 5 years. Over two-thirds of these
deaths, often associated with inappropriate feeding practices, occurred during
the first year of life1. The infant mortality rate of India is 35.2
per 1,000 live births. As per NFHS-5, in India, 63.7% of children below 6
months of age are exclusively breastfed and 45.9% of children aged 6 to 8
months are receiving solid or semi-solid food and breastmilk2. As
per NFHS-5, 67.1% of children of age 6 to 59 months in India are anemic. Only
11.1% of breastfeeding and 12.7% of nonbreastfeeding (total 11.3%) children
aged 6 to 23 months are receiving an adequate diet2.
Dietary sources of vitamin B12 are almost
exclusively from animal foods. As far as anemia in malnutrition or severe acute
malnutrition (SAM) is concerned much emphasis is laid on supplementation of
iron and folic acid and not on vitamin B123. Moreover,
supplementation of only folic acid in children deficient in both vitamin B12
and folic acid can worsen the neurological status of the child4.
There is a lack of data about SAM and specific micronutrient deficiencies in
India; hence, the present study was aimed to study the iron profile, folic
acid, and vitamin B12 levels, and their correlation with the
clinicoepidemiological profile of children with SAM.
This hospital-based cross-sectional study was
conducted at the Malnutrition Treatment
Center (MNTC), Dept. of Pediatric Medicine of a tertiary care center; children with SAM, aged 6 months to 5 years were included in
the study after receiving the requisite clearance from Institutional Ethics
Committee. Exclusion criteria were children who were already on hematinics
before admission, those aged below 6 months and above 5 years, and refusal
for consent. At 95% confidence level and 5% absolute allowable
error assuming 94% prevalence of anemia among SAM cases, the required sample size was 91 cases, which was further
rounded off to 95 cases of SAM.
A predesigned structured proforma was used
to collect information. Basic demographic data, child’s profile (address,
age, sex, birth spacing, and birth order); education, occupation, and religion
of parents; socioeconomic details of their parents (family income, caste), breastfeeding (immediate breastfeeding, exclusive breastfeeding, duration of feeding) and introduction of complementary food and child feeding status were collected
from all patients. Weight was measured in kilograms using an electronic weighing scale. Mid-upper arm circumference (MUAC) was measured in centimeters using a simple measuring tape. These anthropometric measurements were compared to the WHO reference
standards to determine the nutritional status of the child.
Two milliliters of the peripheral venous blood
sample was taken with aseptic precautions, in an EDTA vial for determination of
complete blood count, 2 mL in the plain vial for C-reactive protein (CRP), and
3 mL in another plain vial for serum iron profile, folic acid, and vitamin B12
estimation. Serum iron profile, folic acid, and vitamin B12 were estimated by
using the electrochemiluminescence (ECL) method using VIT B12 600, FOL III
618, and FERRITIN 381 ELECSYS kits for COBASe411 analyzer, Roche diagnostics
GmBH Germany distributed by Roche diagnostics GmBH, Sandhofer Strasse 116
Mannheim.
Iron deficiency was defined as ferritin concentration
<12 ng/mL, or if the CRP was >5 mg/L, iron deficiency was defined as
ferritin concentration <30 ng/mL.5 Vitamin B12 deficiency was
defined as serum or plasma total vitamin B12 concentrations <148 pmol/L, and
vitamin B12 insufficiency was defined as vitamin B12 concentrations
<221 pmol/L, unless otherwise specified. Folate deficiency was defined as a
serum folate concentration <10 nmol/L6. Biochemical evidence of
inflammation was defined as a CRP concentration >5 mg/L. CRP was
measured to evaluate whether systemic inflammation could account for an
abnormal ferritin concentration7.
Data were collected concerning clinical
examination and history given by mother and reliable attendant. The
quantitative data were expressed as mean and SD
and qualitative data as percentage and proportion. The difference in proportion
was analyzed by the Chi-square test and the difference in means was analyzed by ANOVA. P-value <0.05 was taken as significant. All calculations were done by Microsoft Excel, Primer. SPSS Software [version 21] was used for doing statistical
analysis.
The baseline characteristics of the study
participants are depicted in Figure 1. Out of 95 SAM patients, 39 (41.05%)
were between 6 to 12 months, 39 (41.05%) between 12 to 24 months, 9 (9.47%)
between 24 to 36 months, 4 (4.21%) between 36 to 48 months, and
4 (4.21%) were between 48 to 60 months age group. The mean age of the
participants was 19.74 months. Fifty-three (55.79%) were female and 42 (44.21%)
were male with a sex ratio (F:M) of 1.2:1.
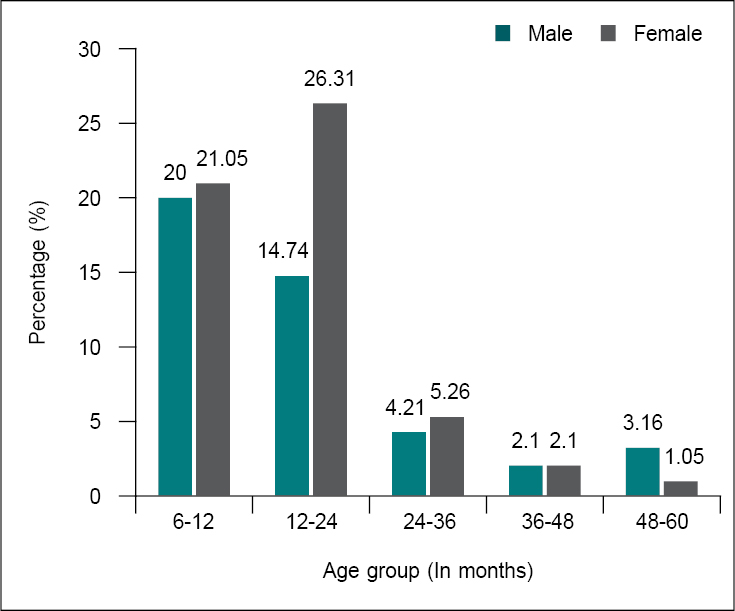
Figure 1. Baseline characteristics of study participants.
Out of 95 SAM children, 75 (78.95%) had
weight-for-height <3SD, 13 (13.68%) patients had weight-for-height <2SD,
and 7 (7.37%) patients had weight-for-height <1SD. Gender-based evaluations
concerning weight-for-height suggested that out of 75 having weight-for-height
<3SD, 33 (34.74%) were male and 42 (44.21%) were female (Table 1).
|
Table 1. Weight-for-Height of Study Participants
|
|
Age group (In months)
|
Weight/Height
|
Total
|
|
<1SD
|
<2SD
|
<3SD
|
|
|
6-12
|
3 (3.16)
|
3 (3.16)
|
32 (33.68)
|
39 (41.05)
|
|
12-24
|
2 (2.10)
|
7 (7.37)
|
30 (31.58)
|
39 (41.05)
|
|
24-36
|
0 (0.00)
|
0 (0.00)
|
9 (9.47)
|
9 (9.47)
|
|
36-48
|
1 (1.05)
|
1 (1.05)
|
2 (2.10)
|
4 (4.21)
|
|
48-60
|
0 (0.00)
|
2 (2.10)
|
2 (2.10)
|
4 (4.21)
|
|
Total
|
7 (7.37)
|
13 (13.68)
|
75 (78.95)
|
95 (100.00)
|
Eighteen
participants (18.95%) had edema and 77 (81.05%) had no
edema. Distribution according to sex and edema shows that out of the 18
patients with edema, 5 were male, and 13 were female. The Chi-square value was
1.679, degree of freedom was 1, and p-value was >0.05, hence not
significant.
Out of the 95 study subjects, 68.42% (65)
patients had MUAC <11.5 cm, and 31.58% (30) of patients had MUAC >11.5
cm. Twenty-nine patients were male and 36 were female out of the 65 (68.42%)
having MUAC <11.5 cm. Thirteen patients were male and 17 were female out of
the 30 (31.58%) having MUAC >11.5 cm. The Chi-square value was 0.01, the
degree of freedom was 1, and the p-value was >0.05, hence not
significant.
Out of 95, 42 (44.21%) children were exclusive
breastfeeding, 40 (42.11%) were still predominantly breastfed and 13 (13.68%)
were given complementary food besides milk. Out of 95, 24 (25.26%) were
completely immunized, 61 (64.21%) were incompletely immunized and 10 (10.52%)
were unimmunized.
Out of 95, 93 (97.89%) were anemic, 42 (44.21%)
had severe anemia, 39 (41.05%) had moderate anemia, and 8 (8.42%) had mild
anemia. Micronutrient deficiency was found in 56 (58.94%). Serum vitamin B12
deficiency was found in 30 (31.58%) patients, serum folate deficiency in 6
(6.32%) patients, and serum iron deficiency in 20 (21.05%) patients. The
severity of anemia in study participants is depicted in Table 2.
|
Table 2. Severity of Anemia in Study Participants
|
|
Age group
(In months)
|
Normal
|
Anemic
(<11 g/dL)
|
Mild anemic
(10-10.9 g/dL)
|
Moderate anemic (7-9.9 g/dL)
|
Severe anemic (<7 g/dL)
|
Total
|
|
6-12
|
0 (0.00)
|
0 (0.00)
|
5 (12.82)
|
16 (41.02)
|
18 (46.15)
|
39
|
|
12-24
|
2 (5.12)
|
2 (5.12)
|
3 (7.69)
|
16 (41.02)
|
16 (41.02)
|
39
|
|
24-36
|
0 (0.00)
|
1 (11.11)
|
0 (0.00)
|
3 (33.33)
|
5 (55.55)
|
9
|
|
36-48
|
0 (0.00)
|
0 (0.00)
|
0 (0.00)
|
1 (25)
|
3 (75)
|
4
|
|
48-60
|
0 (0.00)
|
1 (25)
|
0 (0.00)
|
3 (75)
|
0 (0.00)
|
4
|
|
Total
|
2 (2.10)
|
4 (4.21)
|
8 (8.42)
|
39 (41.05)
|
42 (44.21)
|
95 (100)
|
Mean ± SD of age and severity of anemia analysis
suggested 18.00 ± 0.00 for normal hemoglobin (Hb) (n = 2), 30.75 ±
17.17 for anemia (n = 4), 11.00 ± 3.32 for mild anemia (n = 8), 16.82 ± 10.86
for moderate anemia (n = 39), 15.40 ± 7.79 for severe anemia (n = 42).
Gender-wise evaluation suggested that out of the
42 (44.21%) severely anemic children, 20 (47.62%) were male and 22
(52.38%) were female, and out of 39 (41.05%) moderately anemic children,
18 (46.15%) were male and 21 (53.85%) were female. The study of birth order of
study subjects showed higher prevalence of severe anemia (42) in the third
birth order followed by second birth order, which was 20 and 13, respectively.
Moderate anemia (39) was more in the second birth order followed by the fourth
birth order, which was 15 and 8, respectively.
Table 3 describes
the relationship of the severity of Hb level with weight-for-height of study
participants; 34 (35.79%) participants with severe anemia had weight-for-height
<3SD. The severity of Hb level analysis concerning feeding history of
study subjects suggested that out of 42 exclusive breastfeeding children, 24
had severe anemia, 15 had moderate anemia, and 2 had mild anemia. Mean ±
SD of serum iron according to severity of Hb level was 89.00 ± 13.00 for normal
Hb (n = 2), 77.50 ± 6.10 for anemic (n = 4), 80.00 ± 29.95 for mildly anemic (n
= 8), 110.00 ± 35.94 for moderate anemic (n = 39), 112.12 ± 37.57 for
severe anemic (n = 42) patients.
|
Table 3. Relationship of Severity of Hb Level with
Weight-for-Height of Study Participants
|
|
Hb
|
Weight-for-Height
|
Total
|
|
<1SD
|
<2SD
|
<3SD
|
|
Normal
|
0 (0.00)
|
0 (0.00)
|
2 (2.10)
|
2 (2.10)
|
|
Anemic
|
0 (0.00)
|
1 (1.05)
|
3 (3.16)
|
4 (4.21)
|
|
Mildly anemic
|
2 (2.10)
|
0 (0.00)
|
6 (6.32)
|
8 (8.42)
|
|
Moderate anemic
|
3 (3.33)
|
6 (6.31)
|
30 (31.58)
|
39 (41.05)
|
|
Severe anemic
|
2 (2.10)
|
6 (6.31)
|
34 (35.79)
|
42 (44.21)
|
|
Total
|
6 (6.31)
|
32 (33.68)
|
57 (60.0)
|
95 (100.00)
|
Out of 95, 20 patients had abnormal iron. Out of
20, 11 (11.58%) patients had MUAC <11.5 cm and 9 (9.47%) patients had MUAC
of >11.5 cm. The Chi-square value was 2.110, the degree of freedom was 1,
and the p-value was >0.05; hence, not significant.
Analysis of severity of Hb level and serum iron
of study subjects suggested that out of 20 children with abnormal serum iron,
10 were severely anemic, 8 were moderately anemic,
and 2 were mildly anemic. Distribution according to age and serum iron shows that 20 patients had abnormal iron. Out of 20, 9 patients were in the 6 to 12 months age group and 6 patients were in the 12 to 24 months age group.
Distribution
according to sex and serum iron shows that 20 patients had abnormal iron. Out
of 20, 9 patients were male and 11 were female. The Chi-square value was 0.01,
the degree of freedom was 1, and the p-value was >0.05; hence, not
significant. Distribution according to serum iron and feeding history shows
that 20 children had abnormal iron. Out of 20, 6 were exclusive
breastfeeding, 12 were still predominantly breastfed, and 2 were given
complementary food besides milk.
Analysis of severity of Hb level and total
iron-binding capacity (TIBC) of study subjects suggested that out of 8 children
with abnormal serum TIBC, 3 were severely anemic, 3 were moderately anemic, and
2 were mildly anemic. According to severity of Hb level, the mean ± SD of TIBC
was 359.00 ± 9.00 for normal Hb (n = 2), 346.75 ± 34.23 for anemic (n = 4),
362.50 ± 53.84 for mildly anemic (n = 8), 307.31 ± 58.37 for moderately anemic
(n = 39), 315.50 ± 59.26 for severely anemic (n = 42) patients (Table 4).
|
Table 4. Relationship Between Severity of Hb Level as per
Vitamin B12 of Study Subjects
|
|
Hb
|
Vitamin B12
|
Total
|
|
Abnormal
|
Normal
|
|
Normal
|
1 (1.05)
|
1 (1.05)
|
2 (2.10)
|
|
Anemic
|
4 (4.21)
|
0 (0.00)
|
4 (4.21)
|
|
Mildly anemic
|
0 (0.00)
|
8 (8.42)
|
8 (8.42)
|
|
Moderate anemic
|
15 (15.79)
|
24 (25.26)
|
39 (41.05)
|
|
Severe anemic
|
10 (10.53)
|
32 (33.68)
|
42 (44.21)
|
|
Total
|
30 (31.58)
|
65 (68.42)
|
95 (100.00)
|
Out of 30 children with abnormal serum vitamin
B12, 10 were severely anemic, 15 were moderate anemic, 4 were anemic, and
1 was normal (Table 4).
Analysis of severity of Hb level and serum
ferritin of study subjects suggested that out of 52 children with abnormal
ferritin, 25 were severely anemic, 25 were moderate anemic, and 2 were mildly
anemic. Mean ± SD of serum ferritin according to severity of Hb level was 77.46
± 47.55 for normal Hb (n = 2), 40.18 ± 17.33 for anemic (n = 4), 64.85 ± 45.56
for mildly anemic (n = 8), 192.04 ± 137.95 for moderate anemic (n = 39),
217.10 ± 187.41 for severe anemic (n = 42) patients.
The relationship between severity of Hb level as
per vitamin B12 of study subjects depicted mean ± SD of vitamin B12 of
193.5 ± 7.65 for normal Hb (n = 2), 346.48 ± 258.61 for anemic (n = 4),
323.34 ± 74.71 for mildly anemic (n = 8), 295.29 ± 158.23 for moderately anemic
(n = 39), 266.26 ± 106.56 for severely anemic (n = 42) patients. Out of 95, 30
(30.58%) patients had abnormal vitamin B12 levels. Out of 30, 10 (10.53%) were
in 6 to 12 months age group and 15 (15.79%) were in 12 to 24 months age group,
3 (3.33%) were in 24 to 36 months age group and 2 (2.1%) were in 48 to 60
months age group. Out of 30, 14 were male and 16 were female; Chi-square value
was 0.011, degree of freedom was 1 and p value was >0.05, hence not
significant.
Out of 30, 13 (13.68%) were exclusively
breastfeeding, 10 (10.53%) were still predominantly breastfed, and
7 (7.37%) were given complementary feeds besides milk; Chi-square value
was 3.779, degree of freedom was 2, and p-value was >0.05; hence, not
significant.
Out of 30, 19 (20%) patients had MUAC <11.5
cm and 11 (11.58%) patients had MUAC >11.5 cm. The Chi-square value was
0.530, degree of freedom was 1, and p-value was >0.05; hence, not
significant. Out of 95, 6 (6.31%) children had abnormal serum folate. Out of 6,
2 (2.10%) were severely anemic, 2 were moderately anemic, and 2 were
mildly anemic.
Out of 6 children with abnormal folate, 2 were
in the 6 to 12 months age group, 3 were in the 12 to 24 months age group,
and 1 was in the 24 to 36 months age group. Out of 6, 2 were male and 4 were
female; Chi-square value was 0.017, degree of freedom was 1, and p-value was
>0.05; hence, not significant. Out of 6, 2 were exclusive
breastfeeding, 3 were still predominantly breastfed, and 1 was given
complementary feeds besides milk. Out of 6, 5 had MUAC <11.5 cm and 1
patient had MUAC >11.5 cm. The Chi-square value was 0.128, degree of
freedom was 1, and p-value was >0.05; hence, not significant.
The distribution of micronutrient deficiency in
the groups based on severity of anemia depicted that in the normal Hb (n = 2)
group, 1 child had vitamin B12 deficiency. In anemic group (n = 4), all 4
were vitamin B12 deficient. In mildly anemic (n = 8) group, 2 patients had
serum iron deficiency and 2 patients had folate
deficiency; rest 4 were normal. In moderately anemic group (n = 39), 8
patients had serum iron deficiency, 2 patients had folate deficiency, and 15
patients had vitamin B12 deficiency. In severe anemic (n = 42) group, 10
patients had serum iron deficiency, 10 patients had vitamin B12 deficiency, and
2 patients had folate deficiency. Out of 56 SAM patients with micronutrient
deficiencies, 30 (31.58%) had serum vitamin B12 deficiency, 20 (21.05%) had
serum iron deficiency, and 6 (6.31%) had serum folate deficiency (Table 5).
|
Table 5. Prevalence of Hematopoietic Factor Deficiency in
Relation to Severity of Anemia
|
| |
Iron
|
Folate
|
Vitamin B12
|
|
Normal (n = 2)
|
0 (0.00)
|
0 (0.00)
|
1 (3.33)
|
|
Anemic (n = 4)
|
0 (0.00)
|
0 (0.00)
|
4 (13.33)
|
|
Mildly anemic (n = 8)
|
2 (10)
|
2 (33.33)
|
0 (0.00)
|
|
Moderate anemic (n = 39)
|
8 (40)
|
2 (33.33)
|
15 (50)
|
|
Severe anemic (n = 42)
|
10 (50)
|
2 (33.33)
|
10 (33.33)
|
|
Total
|
20 (21.05)
|
6 (6.31)
|
30 (31.58)
|
Most of the data on SAM from developing
countries is retrospective and descriptive type. The present study was a
hospital-based cross-sectional study. Most of the studies were related to
infectious comorbidities, while only a few studies were related to
micronutrient deficiencies in severely malnourished children.
The present study was undertaken to collect data
on demography and micronutrient deficiencies in our area.
The mean age of the
study population was 19.74 months. In our study, 39% of the patients were in
the age group 6 to 12 months. Another study also found that 50% of SAM patients
belonged to the age group 6 to 12 months8. The high proportion of
SAM patients in the early age group in this region could be due to maternal
malnutrition and delayed introduction of complementary feeding9.
Fifty-three (55.79%) of the patients were female with an F:M ratio was 1.2:1.
Various studies have found that males are nearly equally vulnerable to develop
SAM as females.
In our study, we found a preponderance of
females over males. This may be due to ignorance about the health check-ups and
nutrition of female children; however, no definite causal relationship was
found for female preponderance.
In our study, 75 (78.95%) children had
weight-for-height < –3SD, 40 (42.10%) children had visible severe wasting,
65 (68.42%) had MUAC <11.5 cm, while 18 (18.95%) had bilateral pitting edema
of nutritional origin. So, weight-for-height < –3SD appears to be the most
reliable criteria to identify children with SAM, while bilateral pitting edema
appears to be least reliable. Another study also found similar results that
75.8% of cases had their weight for height < –3SD, 24.03% cases had severe
visible wasting, and 27% had bilateral pitting edema10.
In our study, 24 (25.26%) children were found
completely immunized, 61 (64.21%) were partially immunized, and 10 (10.52%)
were unimmunized. In another study, 42.3% of children were completely immunized
and 52% had partial immunization, while 5.7% of children had no immunization10.
According to NFHS-5, the percentage of children aged 12 to 23 months who have
received all basic vaccinations increased from 44% in 2005-06 to 76.4% in
2019-21. Between 2005-06 and 2019-21, this percentage increased more in rural
areas (from 39% to 76.8%) than in urban areas (from 58% to 75.5%). The
proportion of children who received no vaccinations remained low in both
surveys (5%-6%). In Rajasthan, the percentage of children aged 12 to 23 months
who have received all basic vaccinations increased from 26.5% in 2005-06 to
54.8% in 2015-16 and 80.4% in 2019-212,11. This shows less coverage
of immunization in our study area, which is likely due to ignorance and
unawareness about immunization.
According to modified Kuppuswamy socioeconomic
scale, 74 (77.89%) children in our study belonged to the upper-lower class, 18
(18.95%) belonged to the lower-middle class, while 3 (3.16%) children belonged
to the upper-middle class.
In another Indian study, around 75% of families
belonged to lower socioeconomic status, which was similar to our study10.
In another study, 69.4% of children belonged to the lower social class, 19.4%
to the middle class while 5.6% was of the upper class, which also correlates
with the results of our study12.
In the present study, 42 (44.21%) children were
found to receive exclusive breastfeeding till 6 months of age. In a study by
Kumar et al, 6% of babies were found exclusive breastfeeding10. The
rate of exclusive breastfeeding was more in our study, but weaning does not
start at recommended age, which is a predisposing factor for malnutrition. As
per NFHS-5, in India, 63.7% of children under age 6 months are exclusively
breastfed. About 45.9% of children of age 6 to 8 months are receiving solid or
semi-solid food and breast milk. Only 11.1% of breastfeeding children aged 6 to
23 months are receiving an adequate diet and 12.7% of nonbreastfeeding children
aged 6 to 23 months are receiving an adequate diet. Total children age 6 to
23 months receiving an adequate diet are 11.3 %2.
The overlapping nature of protein-energy
malnutrition and micronutrient deficiencies is well understood and it is seen
that lack of one micronutrient is typically associated with deficiency of
others13.
In the present study, 93 (97.89%) children had
anemia. Out of these 8 (8.42%), children had mild anemia, 39 (41.05%) had
moderate anemia, while 42 (44.21%) children had severe anemia. The high
incidence of anemia in these children could be due to nutritional factors as
well as incidental worm infestations. The high prevalence of anemia was also
reported by other studies10,14. As per NFHS-5, 67.1% of children of
age 6 to 59 months in India are anemic2.
In our study, 56 (58.94%) patients had
micronutrient deficiencies. This could be due to chronic disease, worm infestation,
and protein-energy malnutrition. In the earlier studies on hematopoietic
micronutrient levels in anemic children, iron deficiency was found to be the
commonest, whereas in our study on SAM patients serum vitamin B12 deficiency
was more common (31.58%) compared to iron (21.05%) and folate (6.32%)
deficiency14,15. In the study by Yaikhomba et al, serum vitamin B12
deficiency (34%) was more common than iron and folate deficiencies (6% each) in
SAM patients, similar to this study8.
This could be due
to routine iron and folic acid supplementation to the pregnant and lactating mothers, whereas higher vitamin
B12 deficiency rate could be due to low maternal vitamin B12 levels in a
predominantly vegetarian community
where no vitamin B12 supplementation is routinely given. Vitamin B12 deficiency
is well recognized in
exclusively breastfed infants of vitamin B12-deficient mothers16.
Concentrations of vitamin B12 in breast milk reflect maternal vitamin B12
stores17, and maternal vitamin B12 stores are depleted in up to
one-third of rural Indian women18.
A study in rural Karnataka also found that the
concentration of ferritin and vitamin B12 was decreased in toddlers who
continued to receive breast milk19. Folate concentration in
breast milk is generally high and independent of maternal stores, thus
prolonged breastfeeding in most of our
patients might protect from folate deficiency20. Other studies also
found that 58% and 14.4% of the SAM patients were deficient in vitamin B12
10.
Severe acute malnutrition is an important
preventable and treatable cause of morbidity and mortality in children below 5
years of age in India.
Although malnutrition is highly prevalent in
Indian children, there are very limited data that use biochemical indexes to
characterize the epidemiology of micronutrient deficiencies in children with
SAM in rural communities where the burden is highest.
A detailed understanding of how micronutrient
concentrations relate to factors such as child feeding practices, maternal
health and nutrition, and broader factors such as maternal education and family
wealth and food security, together with a detailed model of how these factors
are related, would enable improved targeting of resources, including
micronutrient supplementation and fortification programs within rural
populations where the majority of India’s population lives.
Malnutrition is
predicted by recurrent hospitalizations, taking an unbalanced diet, lack or
incomplete immunization, and lower socioeconomic status. Immunization coverage
is not enough in our area, as shown in this study that most children were
either partially immunized or unimmunized. So, awareness about immunization
needs to be increased. Strengthening of the infant feeding practices needs to be done by promoting
exclusive breastfeeding for the first 6 months of life, followed by appropriate
complementary feeding with continued breastfeeding. Under-5 children should be
screened for protein-energy malnutrition at the community level for early
diagnosis and prompt management
as a way of reducing the high mortality associated with admitted severe cases.
Nearly all SAM patients have micronutrient deficiencies (Iron, folic acid, and
vitamin B12). So, micronutrient supplement (Iron, folic acid, and vitamin B12)
needs to be given to all SAM patients.
Acknowledgments: We
acknowledge the trust and co-operation of children and their parents who
participated in this study.
Conflict of Interest: None.
Funding: The author(s) received no financial support
for the research, authorship, and/or publication of this article.
1.
Bhadoria AS, Kapil U, Bansal R, Pandey RM, Pant B, Mohan A. Prevalence of severe acute malnutrition and associated sociodemographic factors among children aged 6 months-5 years in rural population of Northern India: a population-based survey. J Fam Med
Primary Care. 2017;6(2):380-5
2.
International Institute for Population Sciences (IIPS) and ICF. National Family Health Survey (NFHS), 2019-21. India: Volume 1. Mumbai: IIPS; 2021.
3.
Mattiello V, Schmugge M, Hengartner H, von der Weid N, Renella R; SPOG Pediatric Hematology Working Group. Diagnosis and management of iron deficiency in children with or without anemia: consensus recommendations of the SPOG Pediatric Hematology Working
Group. Eur J Pediatr. 2020;179(4):527-45.
4.
Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children. Geneva: World Health Organization; 2013. Available from: http://www.ncbi.nlm.nih.gov/books/NBK190328/
5.
Chandra J. Megaloblastic anemia: back in focus. Indian J Pediatr. 2010;77(7):795-9.
6.
Venkatramanan S, Armata IE, Strupp BJ, Finkelstein JL. Vitamin B-12 and cognition in children. Adv Nutr. 2016;7(5):879-88.
7.
Chandelia S, Chandra J, Narayan S, Aneja S, Chawla HM, Sharma S, et al. Addition of cobalamin to iron and folic acid improves hemoglobin rise in nutritional anemia. Indian J Pediatr. 2012;79(12):1592-6.
8.
Yaikhomba T, Poswal L, Goyal S. Assessment of iron, folate and vitamin B12 status in severe acute malnutrition. Indian J Pediatr. 2015;82(6):511-4.
9.
Rao S, Swathi P, Unnikrishnan B, Hegde A. Study of complementary feeding practices among mothers of children aged six months to two years - A study from coastal south India. Australas Med J. 2011;4(5):252-7.
10.
Kumar R, Singh J, Joshi K, Singh HP, Bijesh S. Co-morbidities in hospitalized children with severe acute malnutrition. Indian Pediatr. 2014;51(2):125-7.
11.
International Institute for Population Sciences (IIPS) and ICF. National Family Health Survey (NFHS-4), 2015-16: India. Mumbai: IIPS; 2017.
12.
Ubesie AC, Ibeziako NS, Ndiokwelu CI, Uzoka CM, Nwafor CA. Under-five protein energy malnutrition admitted at the University of Nigeria Teaching Hospital, Enugu: a 10-year retrospective review. Nutr J. 2012;11:43.
13.
Savarino G, Corsello A, Corsello G. Macronutrient balance and micronutrient amounts through growth and development. Ital J Pediatr. 2021;47(1):109.
14.
Thakur N, Chandra J, Pemde H, Singh V. Anemia in severe acute malnutrition. Nutrition. 2014;30(4):440-2.
15.
Devi RU, Krishnamurthy S, Bhat BV, Sahai A. Epidemiological and clinical profile of hospitalized children with moderate and severe acute malnutrition in South India. Indian J Pediatr. 2015;82(6):504-10.
16.
Roumeliotis N, Dix D, Lipson A. Vitamin B12 deficiency in infants secondary to maternal causes. CMAJ. 2012;184(14):1593-8.
17.
Pawlak R, Vos P, Shahab-Ferdows S, Hampel D, Allen LH, Perrin MT. Vitamin B-12 content in breast milk of vegan, vegetarian, and nonvegetarian lactating women in the United States. Am J Clin Nutr. 2018;108(3):525-31.
18.
Behere RV, Deshmukh AS, Otiv S, Gupte MD, Yajnik CS. Maternal vitamin B12 status during pregnancy and its association with outcomes of pregnancy and health of the offspring: a systematic review and implications for policy in India. Front
Endocrinol (Lausanne). 2021;12:619176.
19.
Pasricha SR, Shet AS, Black JF, Sudarshan H, Prashanth NS, Biggs BA. Vitamin B-12, folate, iron, and vitamin A concentrations in rural Indian children are associated with continued breastfeeding, complementary diet, and maternal nutrition. Am J Clin Nutr.
2011;94(5):1358-70.
20.
Dror DK, Allen LH. Overview of nutrients in human milk. Adv Nutr. 2018;9(Suppl 1):278S-294S.